Abstract
Introduction:
Patients with multiple myeloma are mostly older and with co-morbidities. Although clinical trials have confirmed an improved response rate with the addition of anti-CD38 mAbs to the immunomodulatory agents and proteasome inhibitors in patients with multiple myeloma, the safety of such an addition remains a concern. This meta-analysis aims to evaluate the safety and tolerability of anti-CD38 mAbs in both newly diagnosed (NDMM) and relapsed/refractory multiple myeloma(RRMM).
Method:
A systematic search of Pubmed and Cochrane databases was conducted and all randomized controlled clinical trials reporting adverse events (AEs) of anti-CD 38 mAbs, daratumumab (Dara) and isatuximab (Isa) were reviewed. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated by employing the random-effects model, Mantel-Hazel method. Review Manager (RevMan) Version 5.4, Cochrane Collaboration 2020 was used for analysis.
Results:
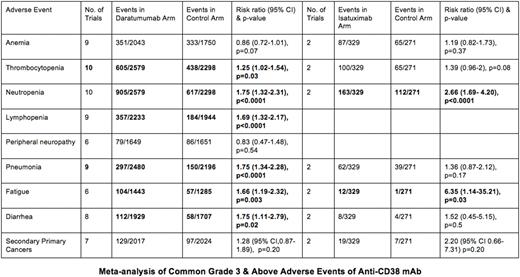
A total of 13 studies (11 Dara and 2 Isa) were identified. Both Isa studies were conducted in patients with RRMM, 5 Dara studies were in RRMM, and 6 Dara studies were in NDMM. The pooled incidences of infusion-related reactions of grade 3 and above were low in both groups, 4.02% in the Dara group and 1.52% in the Isa group. Treatment discontinuation due to adverse events was less common in the Dara group compared to the control group [OR 0.72 (95% CI 0.58-0.92), p=0.009], but the treatment-emergent adverse event (TEAE)-related deaths were not different [OR 0.89 (0.58-1.36), p=0.60]. Similarly, treatment discontinuation was less common in the Isa group compared to control group [OR 0.55 (95% CI 0.32-0.95), p=0.03], but (TEAE)-related deaths were not different [OR 0.88 (0.28-2.72), p=0.82]. The pooled incidences of common grade 3 and above AEs were summarized in the table.
Discussion:
This meta-analysis confirms the safety and tolerability of Anti-CD38 mAbs and highlights the key differences in their AE profiles, with more pronounced thrombocytopenia, pneumonia and diarrhea noted with Dara while both agents resulted in a higher incidence of neutropenia and fatigue. Understanding the AEs of these agents can assist the clinicians in counseling their patients and taking the necessary prophylactic or therapeutic measures to minimize these events. In addition, it can help clinicians in selecting the appropriate agent based on their patients' comorbidities. This study has multiple limitations with the most important one being the low number of Isa clinical trials available to be included in this meta-analysis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal