Abstract
Introduction:
γδT cells target malignant cells by sensing cancer mediated metabolic changes via their γδTCR[1]. We have developed the TEG001 cell product as an 'academic in house product', which are αβ T cells expressing the high affinity Vγ9Vδ2 TCR clone 5 [2]. TEG001 showed tumor reactivity towards a range of malignant cell lines, primary material from patients with various hematological malignancies as well as efficacy in murine models[3]. Here we present the results of dose level 1 and 2, addressing the safety and tolerability of TEG001 in patients with acute myeloid leukemia (AML) or multiple myeloma (MM) (NL6357).
Methods:
The TEG001 study (NL6357), is a single center, investigator-initiated study, with a standard 3+3 dose escalation design. Cohorts receive a single infusion of 1x106 TEG001 cells/kg (dose level 1), 3x106 TEG001 cells/kg (dose level 2) and 1x107 TEG001 cells/kg (dose level 3). Relapsed and/or refractory AML/high risk MDS and MM patients were eligible. AML patients with > 30% blasts or circulating blasts required bridging therapy. TEG001 cells were produced as previously described[2]. The conditioning regimen consisted of fludarabine i.v. 25 mg/m2 (day -4 to -2) and cyclophosphamide i.v. 900 mg/m2 (day -2). Pamidronate (PAM) (30 mg I.V.) was administered on day 0 and on day 28 to propagate TEG001 activity. TEG001 is detected in peripheral blood samples by flow cytometry as part of immune monitoring (details in Fig. 2). The primary endpoint was the development of dose-limiting toxicities (DLTs) in order to determine the maximum tolerated dose. Main criteria for reaching DLTs were TEG001 related adverse events (AEs) requiring ventilator support and grade 4 AEs not related to the underlying disease. AEs contributable to cytokine release syndrome (CRS) and/or immune effector cell-associated neurotoxicity syndrome (iCANS) grade 3 responding to tocilizumab and/or steroids were not defined as a DLT.
Results:
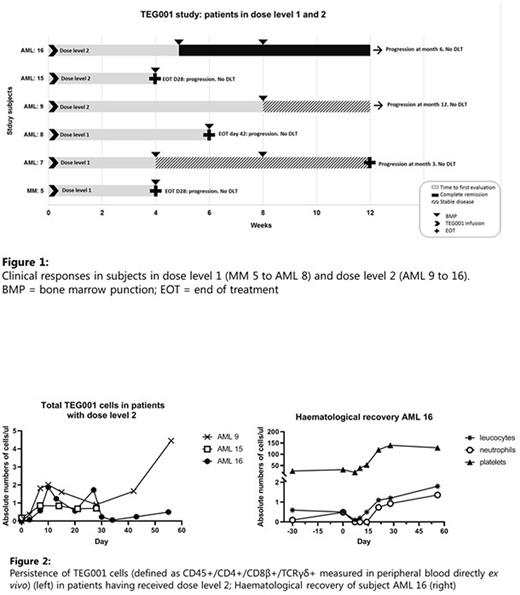
Fourteen patients were included. Of the six patients that were not infused, three patients did not respond to bridging chemotherapy, for two patients TEG001 product could not be manufactured for the intended target dose level and one patients went off study due to COVID-19 induced hospital restrictions. Eight patients were dosed: three patients in dose level 1 and three patients in dose level 2. Two patients received an 'out of specification' dose (77% of dose level 1; and 36% of dose level 2, respectively). Of the six patients infused in dose level 1 and 2, five patients had AML and one patient had MM (Fig. 1). No DLTs were observed. No AEs > grade 3 related to TEG001 were observed. Patient 16 (dose level 2), who received PAM according to the protocol on day 0 and 28, developed 2 seizures at day 29 and 30. The seizure responded well to levetiracetam and no other neurological signs associated with iCANS were observed. No infections or abnormalities in the spinal fluid or brain imaging were identified and connection of the seizure to TEG001 remained inconclusive. In all patients TEG001 cells were detectable in peripheral blood by flowcytometry (dose level 2 depicted in Fig. 2). In dose level 1, patient 7 had stable disease (SD, duration 3M). In dose level 2, patient 9 had a blast reduction (not meeting criteria for partial remission (PR); duration 12M) and patient 16, who had 28% bone marrow blasts prior to TEG001 infusion showed full hematological recovery (Fig. 2) and achieved a complete remission (CR, duration 6M).
Conclusion:
TEG001 cells are well-tolerated at the first 2 dose levels tested. TEG001 cells show persistence up to day 56. One out of three AML patients in dose level 2 achieved a CR one month after TEG001 infusion and SD was observed in both dose levels. The TEG001 study is currently recruiting patients for dose level 3 (1x107 cells/kg).
This study was financially supported by Gadeta b.v.
[1] Sebestyen, Z., et al., Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov, 2019.
[2] Straetemans, T., et al., GMP-Grade Manufacturing of T Cells Engineered to Express a Defined gammadeltaTCR. Front Immunol, 2018. 9: p. 1062
[3] Johanna, I., et al., Evaluating in vivo efficacy - toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J Immunother Cancer, 2019. 7(1): p. 69.
Disclosures
Kuball:Novartis: Research Funding; GADETA: Current equity holder in private company, Patents & Royalties: on gdTCR engineering strategies and targets , Research Funding; Miltenyi Biotech: Patents & Royalties: novel CAR T and engineering isolation strategies, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal