Abstract
Background: Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are well-recognized complications of chimeric antigen receptor (CAR)-T cell therapies. Tocilizumab with or without corticosteroid (e.g., dexamethasone) is the current standard management for moderate to severe CRS (Brudno JN & Kochenderfer JN, 2020 Blood Rev.). However, little is known about the treatment in tocilizumab non-responding CRS patients or in patients with co-occurring ICANS. Using electronic health records (EHRs), we aim to investigate characteristics of CRS induced by CAR-T cell therapies and treatment patterns for CRS in real-world settings.
Methods: We extracted all patients who received CAR-T cell therapies from 5437 multiple myeloma (MM), 1120 diffused large b-cell lymphoma (DLBCL), and 1036 acute lymphoid leukemia (ALL) patients in Sema4 EHRs. We followed the trajectories of CRS and/or ICANS treatments from the initial CRS/ICANS onset after CAR-T cell therapies, through the progression with grade evaluation, until they were resolved. The data was curated automatically, annotated manually and further analyzed descriptively by two domain experts.
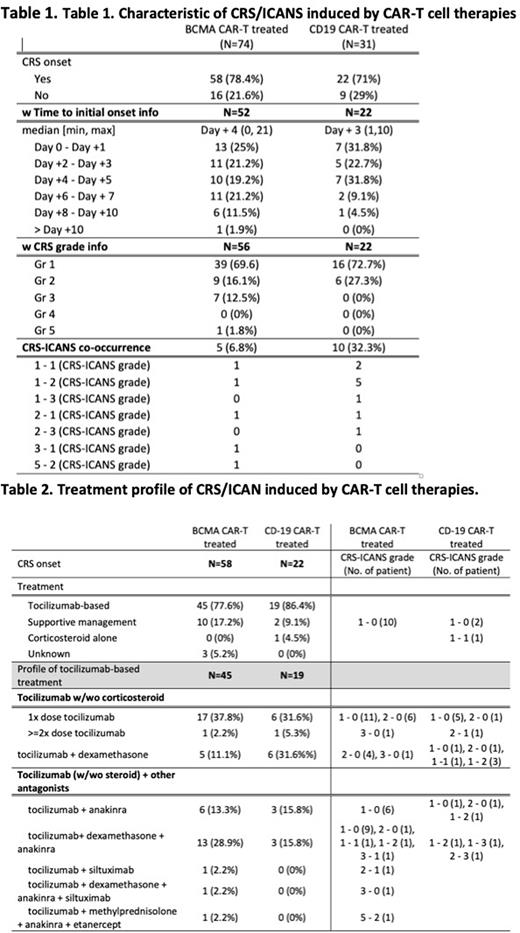
Results: We curated 74 BCMA CAR-T cell treated MM patients and 31 CD19 CAR-T cell treated DLBCL/ALL patients. Among those, 58 (78.4%) BCMA CAR-T treated MM patients, and 22 (71%) CD19 CAR-T treated DLBCL/ALL patients developed CRS. The median initial CRS onset day was Day +3 (min day 0, max day +10) after CAR-T cell infusion (day 0) in CD19 CAR-T cell treated cohorts and Day +4 (min day 0, max day +21) in BCMA CAR-T cell treated cohorts. At Day +5, 86.3% of CD19 CAR-T treated patients and 65% of BCMA CAR-T treated patients developed CRS (Table 1). In CD19 CAR-T cell treated cohorts, 72.7% and 27.3% of patients developed grade 1 and 2 CRS, respectively. Ten patients (32.3%) had co-occurred ICANS. In BCMA CAR-T cell treated cohorts, 69.1%, 16.1%, and 12.5% of patients developed grade 1, 2, and 3 CRS, respectively. Five patients (6.8%) had co-occurring ICANS, and one patient was dead caused by CRS complication (Table 1).
The CRS treatment pattern was summarized in Table 2. As expected, tocilizumab (IL6 receptor antagonist)-based treatment was predominant in both BCMA CAR-T treated (77.6%) and CD19 CAR-T treated (86.4%) groups. Only 17.2% and 9.1% of patients received supportive managements with antibiotics or analgesics. Tocilizumab 1x dose was the most common treatment (37.8% in BCMA CAR-T treated cohorts vs 31.6% in CD19 CAR-T treated cohorts). In CRS grade 2/3 cohorts or ICANS co-occurred cohorts, either the tocilizumab dose was increased (2.2% vs 5.3%), or dexamethasone (corticosteroid) was added (11.1% vs 31.6%) in both BCMA CAR-T treated and CD19 CAR-T treated, respectively. Among the patients who had additional antagonists after tocilizumab, anakinra (IL1 receptor antagonist) was the only antagonist used in CD19 CAR-T cell treated cohorts. Total 6 patients (31.6%) had anakinra after tocilizumab. Patients receiving anakinra had higher rates of ICNAS co-occurrence than patients who did not receive anakinra (67% vs. 38%). In BCMA CAR-T treated cohorts, anakinra was used in 21 (46.6%) patients. The usage of siltuximab (anti-IL6 antibody) was observed in one patient with high CRS grade alone (2.2%, wo anakinra) and one patient with co-occurring ICANS (2.2% w anakinra). Out of 21 anakinra treated patients, 4 (20%) patients had the co-occurrence of ICANS, and 16 (80%) patients had no co-occurrence of ICANS (Table 2). Interestingly, 15 patients complicated with grade 1 CRS only had anakinra in BCMA CAR-T treated cohorts with the description of persistent febrile and increased ferritin level under tocilizumab. Etanercept (TNF antagonist) was also used after tocilizumab, methylprednisolone, and anakinra in one patient with severe CRS complication and co-occurring ICANS.
Conclusions: In this study, we provided a real-world clinical pattern for patients with tocilizumab non-responding CRS or patients with co-occurring ICANS. Gaining a better understanding of toxicity management in those groups can help guiding the treatment of CAR-T cell therapy toxicity and improving quality of life for patients with cancer.
Disclosures
Lee:Sema4: Current Employment, Current equity holder in publicly-traded company. Paek:Sema4: Consultancy. Ai:Sema4: Current Employment, Current equity holder in publicly-traded company. Liu:Sema4: Current Employment, Current equity holder in publicly-traded company. Jin:Sema4: Current Employment, Current equity holder in publicly-traded company. Li:Sema4: Current Employment, Current equity holder in publicly-traded company. Jun:Sema4: Current Employment, Current equity holder in publicly-traded company. Higashi:Sema4: Current Employment, Current equity holder in publicly-traded company. Onel:Sema4: Current Employment, Current equity holder in publicly-traded company. Oh:Sema4: Current Employment, Current equity holder in publicly-traded company; GSK, Janssen, Merck, Pfizer: Consultancy. Pan:Sema4: Current Employment, Current equity holder in publicly-traded company. Stolovitzky:Sema4: Current Employment, Current equity holder in publicly-traded company. Schadt:Sema4: Current Employment, Current equity holder in publicly-traded company; Berg: Consultancy. Wang:Sema4: Current Employment, Current equity holder in publicly-traded company.
OffLabel Disclosure:
Anakinra, Siltuximab and or etanercept for CAR T cell therapy induced CRS treatment
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal