Abstract
Background: The therapeutic landscape for multiple myeloma (MM) has evolved considerably over the last decade and has led to significant improvements in patient outcomes. However, the optimal use of available therapies is not defined and their uptake and use can be limited by regulations, infrastructure or funding, as observed across the Asia-Pacific (APAC) region. Cancer registries play an integral role in generating real world data for monitoring and evaluating current patient management strategies and benchmarking patient care against international standards. The APAC Myeloma and Related Diseases Registry (MRDR) was established in 2018 to collect and analyse data on treatment and outcomes (both survival and quality of life) of MM in the APAC region for the purpose of benchmarking and practice improvement.
Aims: Analysis of preliminary data from October 2018 to July 2022 from the APAC MRDR.
Methods: The APAC MRDR prospectively collects observational data on newly diagnosed MM (NDMM) and other related diseases such as monoclonal gammopathy of undetermined significance (MGUS). Local clinical context and registry oversight is provided by clinical experts from participating countries. Participating hospitals obtain local ethics approval, patient recruitment, and manage data collection. Data collected includes patient characteristics, diagnoses, medical history, treatment (including supportive therapies), and outcomes (overall and progression-free survival, and quality of life using the EQ-5D-5L). Participants are reviewed 4-monthly for a minimum of 5 years.
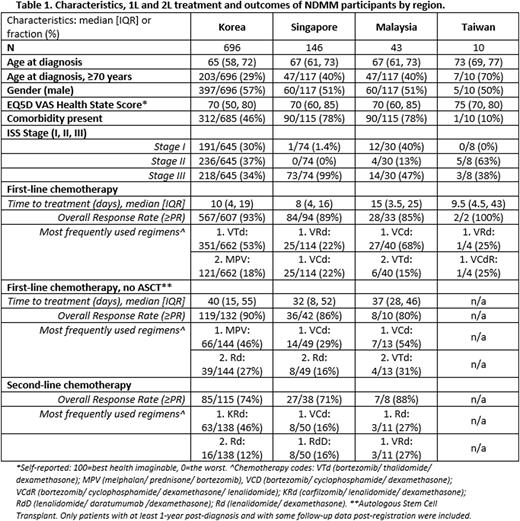
Results: 22 hospitals have ethics approval to participate and patient recruitment has commenced at 19 hospitals in Korea, Singapore, Malaysia, and Taiwan. Additional hospitals, including those in China, have ethics applications in progress. To date, 1118 participants have been registered and, of those, 864 NDMM participants from Korea, Singapore, Malaysia, and Taiwan were available for analysis. Results are presented in Table 1.
Summary and Conclusions: The APAC MRDR registry is now established, and as cases and data accrue, the registry provides a better reflection of clinical practice and patient outcomes. It is a critical tool to identify gaps and highlight challenges in MM patient management within the APAC region.
Disclosures
Aoki:Janssen-Cilag: Research Funding. Wood:Amgen: Research Funding; Astra Zeneca: Research Funding; Abbvie: Research Funding; Sanofi: Research Funding; Antengene: Research Funding; Beigene: Research Funding; Bristol-Myers Squibb: Research Funding; CSL Behring: Research Funding; Gilead: Research Funding; Janssen-Cilag: Research Funding; Novartis: Research Funding; Roche: Research Funding. McQuilten:Sanofi: Research Funding; Novartis: Research Funding; Janssen: Research Funding; GSK: Research Funding; BMS/Celgene: Research Funding; CSL: Research Funding; Beigene: Research Funding; Abbvie: Research Funding; Amgen: Research Funding; Takeda: Research Funding. Chng:Hummingbird: Research Funding; Takeda: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; BMS: Honoraria; Celgene: Honoraria, Research Funding; J&J: Honoraria, Research Funding; Amgen: Honoraria. Spencer:Haemalogix: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal