Abstract

We report a first-in-human gene therapy strategy to extend telomere length in patients with telomere biology disorders. Patients with telomere biology disorders, including dyskeratosis congenita, have extremely short telomeres leading to premature cellular senescence and increased risk of bone marrow failure. Zinc Finger and SCAN Domain Containing 4 (ZSCAN4) is a protein that regulates telomere elongation, enhances genome stability, and is expressed transiently in preimplantation embryos, germ cells during meiosis, embryonic stem cells, and rare adult tissue stem cells.
EXG-001 is a non-integrating temperature-sensitive non-transmissible Sendai virus vector encoding for human ZSCAN4. We demonstrated that the transient exposure to ZSCAN4 via EXG-001 extended telomeres of primary fibroblast cells derived from dyskeratosis congenita patients as well as healthy human CD34+ cells. However, we did not know whether EXG-001 can extend short telomeres of CD34+ cells of dyskeratosis congenita patients.
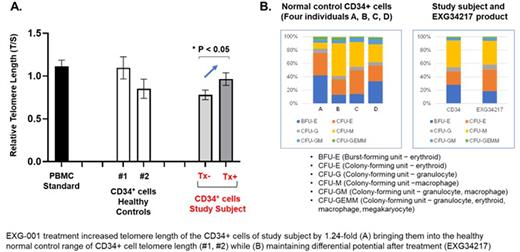
We translated this work in a first-in-human clinical trial (NCT04211714), in which autologous CD34+ hematopoietic stem cells (HSC) were collected by pheresis from an adult subject with dyskeratosis congenita over two days after mobilization with plerixafor and filgrastim with IRB approval and consent. 2.8x106 CD34/kg were collected and treated with EXG-001 ex vivo in a functionally closed tubing system using the CliniMACS Prodigy (Miltenyi Biotec). EXG-001 treatment increased telomere length of the CD34+ cells of study subject by 1.24-fold, bringing them into the healthy normal control range of CD34+ cell telomere length (Figure 1A). To address the concern of effects on terminal colony forming unit studies were performed and demonstrated maintenance of normal differentiation potential after treatment (Figure 1B; EXG34217). 0.85x106 CD34/kg autologous treated HSC (EXG34217) were reinfused into the subject in February 2022 without conditioning regimen and further in vivo studies are in progress.
This novel approach is genotype and mutation independent and uses modified autologous stem cells in the absence of conditioning regimen or immunosuppression which is highly desirable in this radiation and chemotherapy sensitive population. These data support further investigation and translation of telomere elongation via ZSCAN4 exposure by EXG-001 in patients with telomere biology disorders as a potential therapeutic intervention for the prevention or treatment of bone marrow failure.
Disclosures
Davies:Elixirgen Therapeutics: Other: Industry sponsored Clinical trial research . Lutzko:Elixirgen Therapeutics: Other: Industry sponsored Clinical trial research . Wahle:Elixirgen Therapeutics: Other: Industry sponsored Clinical trial research . Aubert:Repeat Diagnostics: Current Employment; Elixirgen Therapeutics: Other: Repeat Diagnostic contracted for clinical trial testing. Amano:Elixirgen Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Amano:Elixirgen Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Yu:Elixirgen Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Ko:Elixirgen Therapeutics: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal