Abstract
Platelets are one of the most metabolically active cells in the bloodstream. They show metabolic flexibility, switching between glycolysis and oxidative phosphorylation (OxPhos) depending on oxygen tension and the availability of substrates. Platelets also have metabolizable glycogen granules that contribute to the energy pool upon activation. Our goal was to investigate platelet energy metabolism under normal physiological conditions to better understand the relative roles of the major ATP-generating processes (glycolysis, OxPhos, and glycogenolysis).
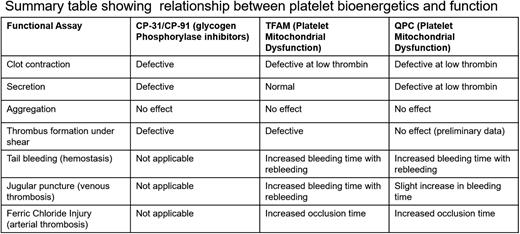
To address the role of glycogen, we used 2 glycogen phosphorylase inhibitors and tested their effects on standard platelet functions e.g., secretion, aggregation, clot contraction, and thrombus formation under shear. We also measured the glycogen levels in resting and activated platelets and the presence of inhibitors. Consistent with previous studies, glycogen was utilized when platelets are activated with a ~25% decrease upon thrombin-mediated activation. Both inhibitors blocked the use of glycogen and led to a 3-fold increase in glycogen compared to controls. These data suggest the active turnover of glycogen in platelets. Our inhibitor studies indicated that glycogen is dispensable for low-energy processes like aggregation but contributed to secretion, clot contraction, and thrombus formation under shear. The inhibition was rescued by either increasing external glucose or bypassing glycolysis with added pyruvate. This study shows that the glycogen granules are metabolically active and contribute to the energy pool upon platelet activation; this pool is important for high-energy platelet functions like clot contraction.
Deletion of platelet glucose transporters (GLUT1 and GLUT3) revealed an essential role of glucose metabolism in hemostasis in vivo. Glucose can power platelets via glycolysis alone or in combination with OxPhos (in the mitochondria). We sought to probe the relative importance of platelet mitochondrial bioenergetics in hemostasis and thrombosis. Previously used mitochondrial inhibitors (antimycin, oligomycin) are toxic and cannot be used for in vivo studies, thus we developed two novel mouse models with altered mitochondrial function using a platelet-specific deletion of TFAM and QPC. TFAM (Transcription Factor A Mitochondrial), is essential for the maintenance, transcription, and translation of mitochondrial DNA. Its deletion is expected to disrupt platelet mitochondrial DNA, which encodes 13 subunits of OxPhos. QPC is a subunit of ubiquinol-cytochrome c reductase complex III. Its deletion is expected to disrupt Complex III, which is part of the platelet mitochondrial respiratory chain.
We confirmed TFAM deletion using Western blot analysis. Using the Seahorse Analyzer, we confirmed that deleting TFAM disrupted platelet mitochondrial bioenergetics in both resting and thrombin-stimulated platelets as seen as a ~40% decrease in Oxygen Consumption Rate (OCR). We also observed a significant decrease in ATP production indicating the contribution of OxPhos to ATP generation. Aggregation was not affected but in vitro clot contraction and thrombus formation under shear were defective (~58% increase in lag time, ~30% increase in AUC, and ~60% decrease in surface area coverage). Both the KO animals showed an increased tail-bleeding time, an increased occlusion time in the FeCl3 carotid injury model, and delayed hemostasis in a jugular puncture injury model with significantly higher rebleeding indicating that mitochondrial bioenergetics is important for clot stability. Using two novel mouse models with dysfunctional mitochondrial bioenergetics, we show that OxPhos is dispensable for low-energy demanding platelet functions such as aggregation but is important for secretion, clot contraction, hemostasis, and thrombosis (Table 1).
Platelets show considerable plasticity in energy metabolism using both glycolysis and oxidative phosphorylation. Using mouse models and inhibitors, we show the relative importance of these energy-producing processes and the fuels and metabolic pathway choices made by platelets at different functional stages. Improving our understanding of platelet metabolism and the relative contributions of each pathway in the future could lead to a better understanding of how metabolic disorders can affect thrombotic risk in diabetic and obese patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal