Abstract
Background: Clonally acquired UBA1 mutations have recently been recognized as the cause of an inflammatory-hematologic overlap disease called VEXAS (vacuoles, E1, X-linked autoinflammatory, somatic) syndrome. Patients with VEXAS syndrome display bone marrow failure similar to myelodysplastic syndrome (MDS), and in addition have severe systemic inflammation. UBA1 is the E1 enzyme required for initiating most ubiquitylation in the cell, and the majority of disease-causing mutations identified occur at p.Met41, the start codon for the cytoplasmic isoform of UBA1 (UBA1b). These mutations lead to an isoform swap with loss of active UBA1b and emergence of a new catalytically impaired isoform, UBA1c, initiated from p.Met67. Despite extensive investigation into the clinical manifestations of UBA1, little is known about the mechanism of disease.
Methods: We performed exome sequencing on 183 patients with clinical manifestations similar to VEXAS syndrome, but without an identified pathogenic mutation on targeted testing. Catalytic activity profiling was performed in vitro and in cells on candidate disease-causing variants identified, assessing for UBA1 thioester formation, E2 transthiolation, and overall cellular ubiquitylation. UBA1 mutations from lung cancer and SMA were also assessed.
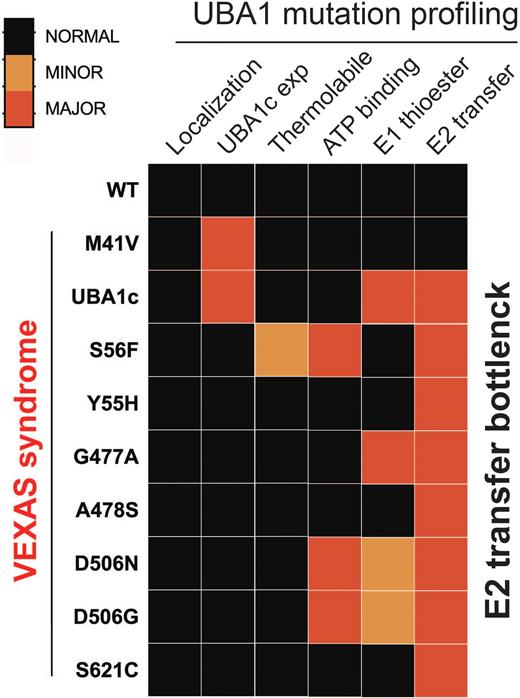
Results: Here we report the identification and molecular dissection of six novel somatic mutations in UBA1 (p.His55Tyr, p.Gly477Ala, p.Ala478Ser, p. Asp506Gly, p.Asp506Asn, and p.Ser621Cys) across nine individuals that lead to VEXAS syndrome. The majority of these mutations were identified through unbiased screening of exome sequencing data of patients with clinical manifestations similar to VEXAS syndrome but without a known pathogenic mutation. Patients with these mutations have highly overlapping symptoms with patients carrying canonical mutations. Specifically, all patients were male with a median age of disease onset of 75 years of age, had macrocytic anemia, elevated inflammatory markers along with rheumatic diagnoses, were steroid dependent and 3/5 with available bone marrow biopsies had MDS diagnoses. We systematically characterized these UBA1 mutations, along with two other previously reported pathogenic mutations (p.Met41Val, p.Ser56Phe), both in vitro and in cells. We found that only p.Met41 mutations led to a loss of UBA1b levels and production of UBA1c, while all other tested variants did not alter isoform expression or subcellular localization. Instead, these mutations reduce catalytic function of both cytoplasmic and nuclear isoforms by 40 to 90 % and can be subdivided into classes according to their impact on catalysis. Three mutations affect UBA1 function by interfering with ATP binding, one mutation renders UBA1 activity thermolabile, and three mutations specifically impair the transfer of ubiquitin from UBA1 to the E2 enzyme, while not affecting UBA1 ubiquitin thioester formation. This bottle neck in E2 charging is also present in UBA1 mutations driving lung cancer in never smokers (LCINS), but not in UBA1 mutations causing spinal muscular atrophy (SMA). Additionally, we identify E2-specific defects with distinct UBA1 mutations.
Conclusions: Thus, somatic UBA1 mutations leading to cancer or inflammatory-hematologic phenotypes have a similar molecular mechanism of disease, which is different from germline SMA-causing UBA1 mutations. Together, we have expanded the genetic causes of VEXAS syndrome, identified shared properties of pathogenesis, and identified methods to screen variants of uncertain significance in disease.
Disclosures
Patnaik:Kura Oncology, Stem Line Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal