Abstract
Background: CPX-351 (United States: Vyxeos®; European Union/United Kingdom: Vyxeos® liposomal) is a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio. CPX-351 is approved for the treatment of newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes in patients who are candidates for intensive chemotherapy (IC) and aged ≥1 year in the United States and adults in the European Union/United Kingdom. However, the appropriate dosage of CPX-351 in patients unfit for IC may be different from the label dosage. Venetoclax (VEN; BCL-2 inhibitor) + low-dose cytarabine has demonstrated efficacy in unfit patients with AML, and drug synergism/additivity in preclinical studies provided a rationale for combining CPX-351 + VEN. Our analysis evaluates the safety and efficacy of lower-intensity CPX-351 + VEN, based on disease risk classification, in adults with newly diagnosed AML (primarily de novo AML) who are unfit for IC.
Methods: This is an open-label, phase 1b study (NCT04038437). Patients who achieve at least partial remission after 1 or 2 cycles may receive up to 4 similar cycles of CPX-351 + VEN in the dose-exploration phase or up to 8 similar cycles in the expansion phase. Patients received CPX-351 dose levels of 20 units/m2 (dose level 1; n = 4), 40 units/m2 (dose level 2; n = 7), or 30 units/m2 (dose level 1b [recommended phase 2 dose]; n = 24) intravenously on Days 1 and 3 in combination with VEN 400 mg/day orally on Days 2 to 21 of each 28-day cycle. Patients are assessed for response (morphology, measurable residual disease [MRD]) and monitored for safety and survival. Patients were categorized into disease risk subgroups based on National Comprehensive Cancer Network criteria.
Results: This analysis included data from 35 patients enrolled across dose levels as of the data cutoff date of March 23, 2022, including 16 (46%) with favorable/intermediate and 19 (54%) with poor disease risk. Of the patients with favorable/intermediate disease risk, median age was 74 (range: 60, 90); 50% were male; 81% had a diagnosis of de novo AML. Of the patients with poor disease risk, median age was 74 years (range: 59, 89); 68% were male; 74% had a diagnosis of de novo AML; and 42% had mutated TP53. Patients were considered unfit for IC based on age ≥75 years (n = 8 [50%] for favorable/intermediate and n = 9 [47%] for poor disease risk) or ECOG performance status of 2 or 3 or comorbidities (n = 8 [50%]; n = 10 [53%]).
Treatment-emergent adverse events (TEAEs) in ≥25% of patients with favorable/intermediate disease risk included febrile neutropenia (38%) and neutropenia (25%) and in those with poor disease risk included nausea (42%), febrile neutropenia (37%), thrombocytopenia (32%) and diarrhea (26%). The majority of patients with favorable/intermediate (94%) or poor (79%) disease risk experienced a grade ≥3 TEAE, primarily myelosuppression. Serious AEs were experienced by 11/16 (69%) and 14/19 (74%) patients, respectively, and the most common serious AE was febrile neutropenia (25% and 37%). There were no deaths in either group on or before Day 30; early mortality by Day 60 was 12% and 11%, respectively.
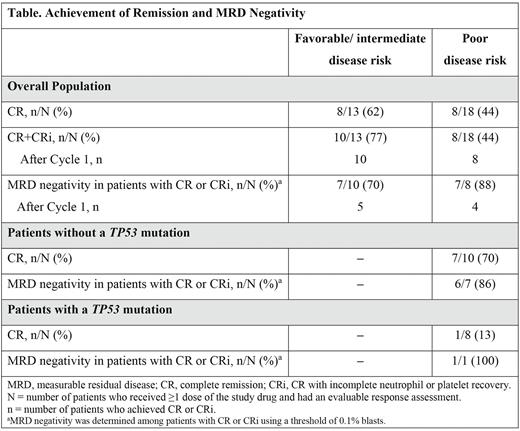
Among patients with an evaluable remission assessment, complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi) was achieved by 10/13 (77%) patients with favorable/intermediate and 8/18 (44%) patients with poor disease risk (Table); all patients who achieved CR or CRi did so after Cycle 1. Among evaluable patients with poor disease risk, 1/8 (13%) with and 7/10 (10%) without a TP53 mutation achieved CR. In patients with CR or CRi, MRD negativity was achieved by 7/10 (70%) patients with favorable/intermediate and 7/8 (88%) with poor disease risk; of these, 5 and 4 patients achieved MRD negativity after Cycle 1, respectively. Median (interquartile range) recovery times to neutrophils ≥500/μL and platelets ≥50,000/μL, respectively, were 30 (28, 36) and 21.5 (21, 28) days for patients with favorable/intermediate disease risk in CR or CRi and 32.5 (21, 38) and 21 (21, 28.5) days for those with poor disease risk in CR or CRi.
Conclusion: The combination of lower-intensity CPX-351 + VEN demonstrated efficacy in both the favorable/intermediate and poor disease risk subgroups, without excessive myelosuppression. These data also provide further insights into outcomes on CPX-351 combination therapies for the treatment of AML.
Disclosures
Lin:AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Baratam:ONO Pharmaceutical: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Stuart:Agios, Astellas Pharma, Bayer AG, Incyte, Jazz Pharmaceuticals, Ono Pharmaceutical, and Sunesis Pharmaceuticals: Research Funding; Sunesis Pharmaceuticals: Consultancy, Honoraria. Walter:Kronos Bio, Inc: Consultancy; Kite Pharma, Inc: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Janssen Global Services, LLC: Consultancy; ImmunoGen: Research Funding; GSK: Consultancy; Genentech: Consultancy; Celgene, Inc: Consultancy, Research Funding; Bristol Myers Squibb, Inc: Consultancy; BioLineRx, LTd: Consultancy, Research Funding; Astellas Pharma US, Inc: Consultancy; Arog Pharmaceuticals: Research Funding; Aptevo Therapeutics: Consultancy, Research Funding; Amphivena Therapeutics, Inc: Current equity holder in publicly-traded company; Amgen: Consultancy, Research Funding; Agios: Consultancy, Research Funding; New Link Genetics: Consultancy; Pfizer, Inc: Consultancy, Research Funding; MacroGenics: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Race Oncology LTD: Consultancy; Boston Biomedical, Inc: Consultancy; BerGenBio, ASA: Consultancy; Selvita: Research Funding; Stemline Therapeutics: Research Funding; AbbVie: Consultancy; Orum Therapeutics, Inc.: Consultancy. Winer:Takeda Pharmaceuticals: Consultancy; Abbvie: Consultancy; Novartis, Jazz Pharmaceuticals, Pfizer, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy. Faderl:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Chandrasekaran:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Chakravarthy:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Cheung:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Pullarkat:AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member.
OffLabel Disclosure:
The combination of CPX-351 and venetoclax is considered investigational.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal