Abstract
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a rare lymphoma subtype characterized by proliferation of lymphocyte-predominant (LP) cells associated with a distinct tumor microenvironment. Six major histopathological growth patterns (A to F) have been described by Fan and colls., based on the cellular composition of the tumor microenvironment and its relationship with the LP cells. Group I (patterns A to C) is comprised of nodules rich in small B-cells and follicular dendritic cells and have been associated with low-risk of disease recurrence/progression, while group II (patterns D to F) is rich in small T-cells, lack follicular dendritic cells and have a higher risk of disease recurrence/progression.
Although multiple studies have characterized the tumor microenvironment and its relationship with tumor biology in classic Hodgkin lymphoma and other lymphoma types, these studies are limited for NLPHL.
Regulatory T-cells (Tregs), a highly immunosuppressive subset of CD4+ T cells, represent a key cellular player in the tumor microenvironment by limiting antitumor immune responses. Emerging studies suggest substantial heterogeneity in the immunophenotype and suppressive capacities of Tregs. For example, classic Tregs are positive for CD4, CD25 and FOXP3; however, a highly immunosuppressive subset of Tregs, named Tr1-like cells has been recently identified and characterized by a distinctive immunophenotype (CD4+, EOMES+, GZMK+ but Foxp3- CD25-) and distinct cytokine expression. Tr1-like are highly relevant in cancer because they seem to be clonally expanded in solid tumors and its presence and number correlates with disease progression (Bonnal et al. Nat Immunol, 2021).
Here, we aimed to investigate the Tregs composition (classic and EOMES+ Tr1-like) in the tumor microenvironment of NLPHL and more specifically, to determine cell densities of Tregs in relation with the different histopathological growth patterns of NLPHL. We hypothesize that the number of Tregs will be higher in those immunoarchitectural patterns with the higher risk of disease recurrence/progression.
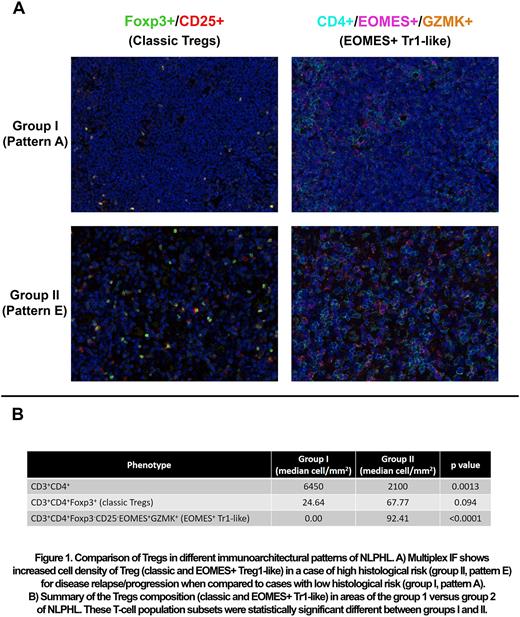
Patients with a diagnosis of NLPHL between 2017 and 2021 with formalin-fixed paraffin embedded tissue biopsies available in our institution were included in the study. All cases were submitted to a previously optimized and validated multiplex IF panel which was specifically developed to assess classic and EOMES+ Tr-1 like Tregs. The panel included CD3, CD4, CD8, CD20, CD25, EOMES, Foxp3, and Granzyme K. Reactive tonsils were used as a positive control. The slides were imaged using Vectra Polaris system (Akoya Biosciences, Marlborough, MA) and up to 10 representative regions of interest (ROI) were selected from each case. The data was consolidated using Spotfire software. The nonparametric Mann-Whitney test was used to compare ROI representing group I and group II histologic areas.
Eleven patients with available pretreatment samples were identified. The majority were male (n=7, 63%), with advanced stage of disease (n=7, 63% Stage III/IV); all patients were alive at last follow-up (median: 42 months). A total of 109 ROIs were analyzed (n=54 in group I and n=55 in group II) with a mean number of 22,723 cells/case, and a mean analyzed area of 1.6 mm2/case. The population of CD4+ T-cells was significantly increased in group I (patterns A-C) when compared to group II (patterns D to F) (median, 640 vs 2100, p=0.0013). Interestingly, the group II was associated with a significantly higher cell density of CD4+ EOMES+ Tr1-like cells when compared to group I (median, 0 vs 1010, p<0.0001) (Figure 1). Although not statistically significant, classical Tregs (CD3+CD4+Foxp3+) were also increased in the group II (median, 24.64 vs 67.77, p=0.094).
In summary, increased cell densities of Tregs and specifically EOMES+ Tr1-like are strongly associated with architectural patterns of high-risk for disease recurrence and progression in NLPHL. Our data provide a biological rational to focus on these subset populations in efforts to decrease disease recurrence and progression in NLPHL.
Disclosures
Steiner:Seagen: Research Funding; BMS: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Pinnix:Merck Inc: Research Funding. Vega:Crisp Therapeutics: Research Funding; Allogene: Research Funding; Geron Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal