Abstract
Introduction: Chimeric antigen receptor T-cells (CAR-T) are widely used for the treatment of relapsed/refractory Diffuse large B cell lymphoma (DLBCL). The data for CAR-T cell therapy in patients with extra-nodal lymphoma is restricted and it is unclear whether short and long term results are comparable in this group with nodal DLBCL and whether outcomes are affected by the different organs involved.
Methods: This is a retrospective, multi-center study conducted in four Bone marrow transplant units across Israel. Our aim was to analyze the response rate and survival in all consecutive patients with DLBCL who were treated with commercially available axicabtagene ciloleucel or tisagenlecleucel between June 2019 and October 2021. We excluded patients with no positron emission tomography - computed tomography (PET-CT) prior to lymphodepletion and patients with primary mediastinum B cell lymphoma. Extra-nodal organs were defined as any organ other than lymph node, Waldeyer's ring, tonsils or spleen. Mediastinal tumors were considered an extra-nodal organ if defined by the radiologist as non-lymph node structure. Response rates, progression free survival(PFS) and overall survival(OS) of patients with extra-nodal disease (END) at lymphodepletion were compared to patients presenting with a nodal disease(ND) only. The END cohort included patients with extra-nodal involvement only or combined nodal and extra-nodal organs.
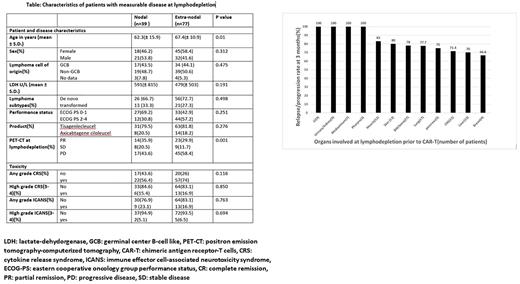
Results: We included 126 consecutive patients with DLBCL treated with commercially available CAR-T cells(tisagenlecleucel, n=100, 79.4% and axicabtagene ciloleucel, n=26, 20.6%). At lymphodepletion therapy prior to CAR-T cells, 77/126(61%) had extranodal disease(END), 39/126(31%) patients had nodal disease(ND) and 10/126(8%) showed no disease assessed by PET-CT. Patients with measurable disease and END were older compared to those with ND (67.4 vs.62.3 years , p= 0.01). All other baseline characteristics of disease and patients were similar between the 2 groups, Table. There was no difference in the incidence of overall cytokine release syndrome(CRS), high grade CRS, overall Immune effector cell related neurotoxicity (ICANS) and high grade ICANS between the 2 groups (all p=NS). There was no difference in the rate of overall response and in the rate of CR at 1 month after CAR-T infusion between END group and ND (57.3% vs 64.1% and 36.3% vs 43.5%, respectively, p=.76). Of note, all the patients with involvement of gastrointestinal tract (n= 9)/urinary tract (n= 9)/ mediastinum (n= 7) or pharynx (n= 3) at lymphodepletion progressed or relapsed less than 3 months from CAR-T administration, Figure.
There was no difference in median PFS between the 2 groups [11.1(95% CI 8.2-14) vs 13.69 (95% CI 9.4-17.9) months, p =0.173)] and the median OS ( 15.9 (95% CI 13.1-18.7) vs. 17.8 (95% CI 14-21.6) months , p =0.182). Subgroup analysis according to the number of END involved sites showed that median PFS was significantly higher in patients with no END [14.6 months (95% CI 10.9-18.3)] or ≤ two END sites at time of lymphodepletion[12.3 months (95% CI 9-15.5)] compared to patients with ≥3 END sites[4.29 months (95% CI 0.5-7.9), p=0.010].
In multivariate cox regression analysis, the number sites of END and high LDH at lymphodepletion were predictive of inferior PFS (p=0.018 and <0.001, respectively), while sex, type of product administered, age and performance status did not predict PFS. Similarly, both LDH and ≥3 END predicted OS (p<.001 and p=.032, respectively), while all other factors did not.
Conclusions: Both short and long outcomes were similar in patients with END and patients with ND-only disease after CAR-T therapy. No increase in toxicity was described in patients with END lymphoma. Patients with involvement of gastrointestinal tract/urinary/kidney/mediastinum or pharynx at lymphodepletion demonstrate grim prognosis. Larger studies should further validate these findings. If confirmed, patients with specific sites of END may be candidate for future trials with post-CAR-T consolidation with immunotherapy strategies.
Disclosures
Herishanu:Janssen, AbbVie, Roche, Medison, BeiGene: Honoraria. Yehudai-Ofir:Gilead: Other: Advisory board 2021; Novartis: Other: Advisory board 2021. Zuckerman:Orgenesis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioSight Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cellect Biotechnology: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Avivi:Kite, a Gilead Company: Speakers Bureau; Novartis: Speakers Bureau. Ram:Takeda: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal