Abstract
Lymphodepleting (LD) chemotherapy prior to adaptive T-cell therapies establishes an immune environment conducive to product expansion and persistence. There is little prospective data regarding the most appropriate regimens to achieve this without exacerbating toxicity. Most lymphodepleting regimens use a combination of fludarabine and cyclophosphamide (flu/cy) but there is variability between dosing and duration of therapy. Outpatient administration of LD chemotherapy and CAR T-cells is growing and has been estimated to decrease overall costs by over 40%. As the number of patients eligible for CAR-T rapidly expands, it will be important to minimize time spent in the infusion center as well as minimize expense. A 2-day lymphodepleting regimen that maintains CAR T-cell efficacy without additional toxicity could allow for more efficient use of resources, decreased costs, and increased patient convenience and satisfaction.
Records of consecutive patients that received CD19 directed CAR T-cell therapy at University of Michigan were retrospectively reviewed. Data regarding patient demographics, LD chemotherapy, CAR T-cell therapy used, toxicities, and outcomes were abstracted. Cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity (ICANS) were defined and graded by ASTCT consensus grading criteria. Persistent neutropenia was defined as an ANC <1000 for 6 weeks. Persistent thrombocytopenia was defined as a platelet count <50 for 6 weeks. Disease related outcomes were defined by best overall response as classified by the Lugano criteria. Data were compared using Fisher's exact and chi-squared tests; however, as no comparative analysis reached statistical significance, results are reported only as descriptive values.
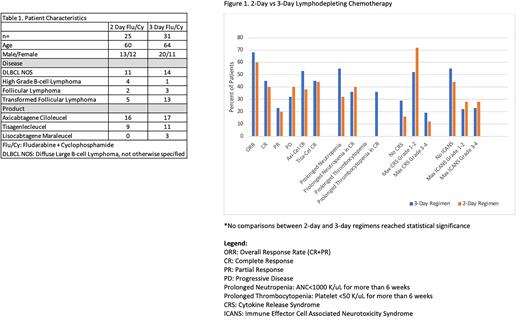
Between June 2018 and June 2021, 56 patients received LD chemotherapy prior to CD19 directed CAR T-cell therapy at our institution. Patients received either the combination of fludarabine 30 mg/m2 + cyclophosphamide 500 mg/m2 for 3 consecutive days on day -4 through day -2 (n=31, 55.3%) or fludarabine 40 mg/m2 + cyclophosphamide 500mg/m2 for 2 consecutive days on day -3 and day -2 (n=25, 44.6%). The baseline patient demographics and distribution of disease types treated with each regimen are summarized in table 1. Of the patients that received 3 days of LD chemo, 54.8% (n=17) were treated with axicabtagene ciloleucel (axi-cel), 11 were treated with tisagenlecleucel (tisa-cel), and 3 received lisocabtagene maraleucel (liso-cel). In those that received 2 days of LD chemo, 16 were treated with axi-cel and 9 were treated with tisa-cel.
In patients receiving a 3-day LD regimen, 29% did not experience CRS, 52% had grade 1 or 2, and 19% developed severe CRS (grade 3-4). In those treated with a 2-day regimen, 16% did not experience CRS, 72% had grade 1 or 2, and 12% developed severe CRS. In patients receiving a 3-day LD regimen, 55% of patients did not experience ICANS, 22% had grade 1 or 2, and 23% developed severe ICANS (grade 3-4). In those treated with a 2-day regimen, 44% did not experience ICANS, 28% had grade 1 or 2, and 28% developed severe CRS.
With a 3-day LD chemotherapy regimen, prolonged neutropenia was noted in 55% of patients and 36% of patients that had a complete response (CR). The 2-day LD chemotherapy regimen showed prolonged neutropenia in 32% of all patients and 40% of patients that reached CR. Prolonged thrombocytopenia was seen in 36% of patients that received the 3-day regimen, but all were in patients that achieved a CR. No prolonged thrombocytopenia was noted with a 2-day regimen.
Patients that received the 3-day LD regimen had an overall response rate (ORR) of 68%. The CR rate was 45% and partial response (PR) rate was 23%. The ORR for patients receiving the 2-day LD regimen was 60%, with a CR rate of 40% and a PR rate of 20%. When comparing across CAR T-cell products, the CR rate for axi-cel was 53% with a 3-day regimen compared to 38% with a 2-day regimen. Patients that received tisa-cel had a CR rate of 45% with a 3-day regimen compared to 44% with a 2-day regimen.
This single center, retrospective study compared 2-day vs 3-day LD chemotherapy regimens before CD19 directed CAR-T therapy. In this small patient sample, there were no significant differences in CAR T-cell efficacy or toxicity with a 2-day regimen. There is need for further prospective trials to ensure appropriate dosing of chemotherapy to optimize CAR T-cell response while minimizing toxicity.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal