Abstract
Introduction: Primary myelofibrosis(PMF), including pre-fibrotic myelofibrosis(Pre-PMF) and overt-fibrotic myelofibrosis(Overt-PMF), may finally transform to the terminal stage, which was defined as primary myelofibrosis-accelerate phase/blast phase(PMF-AP/BP).In the past decade, high-throughput next-generation sequencing(NGS) technologies have been applied to identify a large range of genetic mutations in PMF. However, little is known about the difference in mutational landscape and enrichment of non-driver mutations between Pre-PMF, Overt-PMF and PMF-AP/BP patients, as well as the clonal evolution during disease progression of PMF. Therefore, we aimed to clarify the mutational landscape between Pre-PMF, Overt-PMF and PMF-AP/BP patients as well as the clonal evolution and prognostic effect of non-driver mutations during disease progression.
Methods: This retrospective cohort included 69 Pre-PMF patients,145 Overt-PMF patients and 45 PMF-AP/BP patients from the Institute of Hematology and Blood Diseases Hospital in China from 2015 to 2021. The targeted panel that covered all exons of 137 genes involved in myeloid malignancies or previously described in MPN was used in patients to detect mutations.
X-tile software was used to define a cut-off size of variant allele fractions (VAF) of mutation. ASXL1 mutation VAF≤7.5% was defined as a smaller clone size, while VAF>7.5% was defined as a larger clone size.
Results: A total of 259 patients with Pre-PMF, Overt-PMF and PMF-AP/BP were included in this analysis. Driver mutation distribution was JAK2V617F 55.6%, CALRExon9 24.7%, MPLW515 3.9%,triple-negative 15.8%. Most frequent non-driver mutations were ASXL1 28.6%, TET2 18.9%, SRSF2 11.2%, U2AF1 10.1% and SETBP1 7.7%(Figure 1A-C).
First, we compared the proportion of non-driver mutations between Pre-PMF, Overt-PMF and PMF-AP/BP. Mutations in the ASXL1(30.3%VS10.1%, P=0.001)and U2AF1 (15.2%VS1.4%, P=0.002)genes were more frequent in Overt-PMF compared with Pre-PMF, whereas mutation in the ARID1A (9.1% VS 0.7%, P=0.005)gene was more frequent in Pre-PMF(Figure1D); as mutations in ASXL1(51.1%VS30.3%,P=0.011), SRSF2(26.7%VS9.7%,P=0.004), RUNX1(22.2%VS5.5%,P=0.001), SETBP1(17.8%VS6.2%,P=0.018), NOTCH2(10.8%VS2.2%,P=0.038), NRAS(15.6%VS3.4%,P=0.004)and EZH2(13.3% VS 4.8%,P=0.048) genes were significantly more frequent in PMF-AP/BP compared with Overt-PMF(Figure 1E).
Then, we analyzed the enrichments of non-driver mutations in different subtypes of PMF. ASXL1(OR=4.05,95%CI 1.59~10.31; P=0.003) and U2AF1(OR=12.16,95%CI 1.59~92.22; P=0.002) mutations were enriched in Overt-PMF compared with Pre-PMF, RUNX1 (OR=6.56,95%CI 1.84~23.40; P=0.004)and ASXL1(OR=2.94,95%CI 1.27~6.84; P=0.012) mutations were enriched in PMF-AP/BP compared with Overt-PMF(Figure 1F).
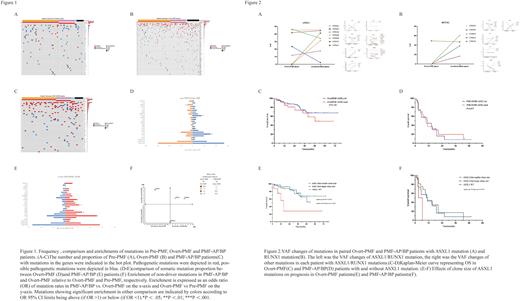
Next, we explored clonal evolution during disease progression in 17 paired sequential samples. Non-driver mutations, especially ASXL1(Figure 2A)and RUNX1(Figure 2B)mutations tended to increase their clone sizes and were more likely to be newly acquired than lost during the transformation from the Overt-PMF phase to accelerate/blast phase. ASXL1 mutation was regarded as an adverse prognostic factor in PMF patients according to previous study. However, in Overt-PMF(Figure 2C) and PMF-AP/BP(Figure 2D) patients, ASXL1 mutation had an insignificant effect on overall survival (OS), so we examined the effect of clone size of ASXL1 mutation on OS. Among Overt-PMF patients with ASXL1 mutation, those with smaller clone size had a significantly shorter OS than those with larger clone size(HR=3.94,95%CI:1.09∽14.25; p=0.024) and those without ASXL1 mutation(HR=4.04,95%CI:1.35∽12.11; p=0.007)(Fig 2E). similarly, Among PMF-AP/BP patients with ASXL1 mutation, smaller clone size was associated with significantly inferior OS (HR=4.05,95%CI: 0.89∽18.47;p=0.045) compared with larger clone size (Figure 2F).
Conclusion: This study clarified the mutational landscape and enrichments of non-driver mutations between different subtypes of PMF patients. The clonal architecture changes and increment of newly acquired pathogenetic mutations were associated with the disease progression of PMF. Smaller clone size of ASXL1 mutation had an adverse prognostic impact in Overt-PMF and PMF-AP/BP patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal