Abstract
Introduction: Over the last decade, covalent Bruton Tyrosine Kinase inhibitors (BTKi) have transformed the therapeutic landscape for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Prior retrospective studies (Maddocks et al. 2015, Hampel et al. 2019) have reported on outcomes for BTKi discontinuation in cohorts of patients from an era when the majority were relapsed or refractory to multiple prior lines of chemoimmunotherapy. Less is known about the outcomes for patients with CLL/SLL who receive BTKi earlier in their treatment course and subsequently discontinue their BTKi. Here, we report on a cohort of contemporary CLL/SLL patients who discontinued BTKi treatment, a majority of whom received index BTKi as first- or second-line therapy.
Methods: We performed a retrospective chart review of adult CLL/SLL patients at Dana-Farber Cancer Institute who were treated with at least one covalent BTKi and found to be treatment resistant/refractory or intolerant to a BTKi. To be included, patients had to have 6 months of records prior to and 12 months of records following index BTKi (i.e., first observed BTKi) initiation. Using a standardized electronic form, data were abstracted from electronic charts including demographics, oncologic and medical histories, clinical variables, treatments initiated, adverse events, reasons for treatment change or discontinuation, and clinical outcomes. Patients who received non-hormonal anti-neoplastic therapy for other primary malignancies between CLL/SLL diagnosis and first BTK inhibitor treatment were excluded from the study.
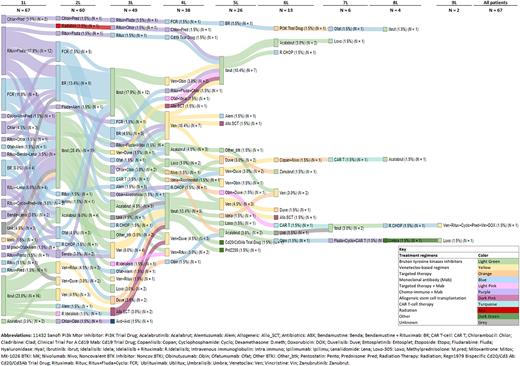
Results: 67 patients who initiated BTKi between 07/2011 and 04/2019 and were later found to be treatment resistant/refractory or intolerant to a BTKi were identified and included in the analysis. 56% of these patients were treated with index BTKi as part of a clinical trial. Median age at first CLL/SLL diagnosis was 58.8 years (Q1-Q3, 53.2-64.8), median age at index BTKi initiation was 66.0 years (Q1-Q3, 60.1-73.1), median time from CLL/SLL diagnosis to index BTKi initiation was 6.9 years (Q1-Q3, 3.3-9.9). Prognostic marker testing revealed: FISH (n=65 assessed) del(13q): 62%, del(11q): 35%, del(17p): 26%, trisomy 12: 25%, complex karyotype (stimulated): 22%; IGHV unmutated (n=50 assessed): 52%, TP53 mutation (n=41 assessed): 56%. Index BTKi treatments were given as: first line (27%), second line (33%), third line (18%), fourth line (12%), fifth or greater line (9%) of therapy (Figure 1); patients were treated with either ibrutinib (88%, 51% as monotherapy and 12% in combination with anti-CD20 mAb) or acalabrutinib (12%, all monotherapy). The duration of index BTKi treatment was a median of 1.7 years (Q1-Q3, 0.6-3.2). 55% of the patients discontinued index BTKi treatment due to adverse events, most commonly atrial fibrillation (22%) and bleeding events (14%); 35% discontinued due to progression of disease, and 5% due to Richter's transformation. Following discontinuation of index BTKi due to adverse events, 19% switched to a second BTKi immediately, and 25% switched to a second BTKi at any point in time. Following last BTKi treatment, 54% of patients received subsequent therapies; among these, 81% received venetoclax-containing regimens. With a median follow-up of 5.4 years from index BTKi initiation to data cutoff, 27 (40%) patients died. Among those with a known primary cause of death (N=21), disease progression (52%), infection (19%), and secondary malignancy (10%) were the most frequently documented causes in the medical record.
Conclusions: In this retrospective study characterizing CLL/SLL patients at a large academic center who received covalent BTKi mostly in early lines of therapy, BTKi discontinuation was most commonly driven by adverse events (55%), with CLL/SLL disease progression (35%) the second most common reason. These findings are consistent with earlier studies, although notably we observed a relatively low incidence of Richter's transformation (5%). Following BTKi discontinuation, many patients received venetoclax-based regimens, yet with a median follow-up of 5.4 years, 40% patients died. This suggests that novel treatment approaches are needed for contemporary patients who discontinue covalent BTKi.
Figure 1. Sankey diagram of treatment sequence for CLL/SLL patients by line of therapy
Disclosures
Huynh:Appellis Pharmaceuticals: Research Funding; Novartis Services, Inc.: Research Funding; Merck & Co., Inc.: Research Funding. Yang:Merck & Co., Inc.: Current Employment. Lema:Merck & Co., Inc.: Research Funding. Matay:Merck & Co., Inc.: Research Funding. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. De Nigris:Merck & Co., Inc.: Current Employment. Gandra:Merck & Co., Inc.: Current Employment. Sarpong:Merck & Co., Inc.: Current Employment. Duh:AstraZeneca: Research Funding; Apellis Pharmaceuticals: Research Funding; Blueprint Medicine: Research Funding; Takeda Oncology: Research Funding; Merck: Research Funding; Novartis: Research Funding; Pharmacyclics: Research Funding; Shire: Research Funding; GSK: Research Funding. Brown:SecuraBio: Research Funding; Grifols: Consultancy; Pharmacyclics: Consultancy; Gilead: Research Funding; MEI Pharma: Consultancy, Research Funding; Janssen: Consultancy; iOnctura: Consultancy; Hutchmed: Consultancy; Genentech/Roche: Consultancy; Eli Lilly: Consultancy, Research Funding; Catapult: Consultancy; Bristol-Myers-Squibb/Juno/Celgene: Consultancy; Beigene: Consultancy, Research Funding; Acerta/ Astra-Zeneca: Consultancy; Abbvie: Consultancy; TG Therapeutics: Research Funding; Sun: Research Funding; Pfizer: Consultancy. Davids:Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy; Novartis: Research Funding; Ono Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Research to Practice: Honoraria; Takeda: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal