Abstract
Background: Ibrutinib is an irreversible inhibitor of Bruton's tyrosine kinase (BTK) widely used in the treatment of CLL. Ibrutinib is associated with cardiac adverse events (AEs) including serious and fatal cardiac arrhythmias, and dose reduction guidelines for cardiac toxicities have recently been published. NIH protocol 15-H-0172 (NCT02514083) is an ongoing phase II study of short-course fludarabine added to continuous ibrutinib in patients with treatment-naïve CLL (N = 29). Following a second case of sudden death on study, we hypothesized that systematic cardiac screening for arrhythmias in clinically asymptomatic patients on ibrutinib could identify individuals at increased risk of cardiac AEs.
Methods: Patients on study received ibrutinib 420 mg orally once daily on 28-day cycles until disease progression or intolerable side effects. Fludarabine 25 mg/m2/day was given intravenously on days 1-5 of cycles three and four. All patients completed combination therapy and are on extension with single-agent ibrutinib. Between February 2021 and December 2021, patients on study (n = 21) underwent protocol-mandated transthoracic echocardiogram, cardiac stress testing (exercise stress n = 20; stress MRI n = 1), and ambulatory monitoring (2-week patch recorder n = 19; 24-hour Holter n = 1; patch recorder discontinued after 24 hours n = 1). Results of the testing were reviewed with a cardiologist and follow-up diagnostics and interventions were performed as clinically indicated.
Results: All patients were asymptomatic at time of testing. As of December 31, 2021, the median follow-up time of enrolled subjects was 54.5 months. The estimated 48-month progression-free survival was 90%.
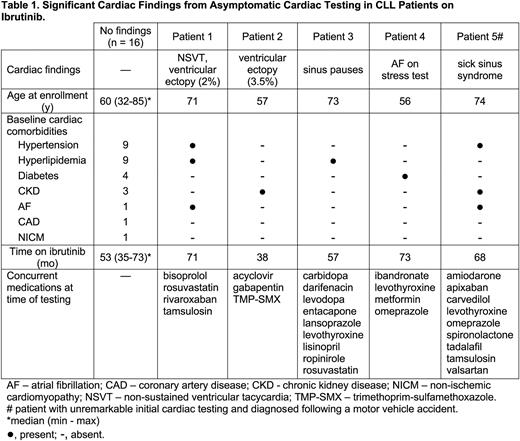
Seventeen (81%) patients had no clinically significant findings on testing and continued ibrutinib without interruption. In four (19%) patients, testing altered therapy: precautionary discontinuation of ibrutinib in two, pacemaker placement in one, and initiation of a beta-blocker for newly diagnosed atrial fibrillation in one (Table 1).
Ambulatory monitoring led to precautionary drug discontinuation in two patients and temporary drug hold in one. Patient 1 had a 2% ventricular ectopy burden and an episode of non-sustained ventricular tachycardia on 24-hour Holter. A 48-hour Holter six weeks after drug discontinuation showed decreased ventricular ectopy (<1%). Patient 2 had a 3.5% ventricular ectopy burden on 2-week patch monitoring. Ventricular ectopy persisted (4.1%) four months after drug discontinuation. Six months after stopping ibrutinib, both patients remain off any CLL therapy and progression-free. Patient 3 had sinus pauses up to 5.6 seconds and second-degree AV block on ambulatory monitoring. Ibrutinib was temporarily held for pacemaker placement.
Stress testing identified new atrial fibrillation not observed on ambulatory monitoring in Patient 4. The patient was initiated on a beta blocker but did not require anticoagulation.
For all patients, there were no unexpected, clinically significant structural abnormalities found by echocardiogram, and no new ischemic heart disease was identified on cardiac stress testing.
Of note, Patient 5 had unremarkable cardiac testing, but three months later presented with syncope and a motor vehicle accident. Ibrutinib was permanently discontinued on admission. The patient was found to have sick sinus syndrome including an episode of bradycardic arrest requiring CPR and underwent pacemaker placement.
Conclusions: Screening for arrhythmias in asymptomatic CLL patients on long-term ibrutinib therapy identified no underlying cardiac conditions in 81% (n = 17) and clinical findings altering management in 19% (n = 4). This cross-sectional assessment was unable to infer or refute a causal role for ibrutinib. However, drug discontinuation was recommended based on these findings in two patients irrespective of their etiology. Despite comprehensive screening efforts, one patient subsequently experienced a serious cardiac adverse event. Further research is warranted to identify efficient monitoring and risk mitigation strategies for cardiac toxicities on ibrutinib and potentially other BTK inhibitors. Future studies should incorporate the recent dose reduction guidelines, pre-treatment cardiac evaluation, and comparison groups, including patients not on treatment and on alternative targeted therapy.
Disclosures
Itsara:Adaptive Biotechnologies: Other: Spouse: current employee and holder of stock options. Sun:Genmab: Research Funding. Haigney:Astrazeneca: Other: Clinical trial endpoint committee; Huxley Medical: Consultancy. Wiestner:Abbvie: Research Funding; AstraZeneca: Research Funding; Acerta Pharma: Research Funding; GenMab: Research Funding; Merck: Research Funding; Nurix: Research Funding; Pharmacyclics: Research Funding; Verastem: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal