Abstract
INTRODUCTION Cancer related distress is an experience comprised of physical, psychological, social, and religious/spiritual factors that impact how a patient (pt) copes with illness. High levels of distress have been associated with increased healthcare utilization. NCCN guidelines recommend pts with significant distress be referred to supportive services (SS) within 6 months of screening. Pts with myeloproliferative neoplasms (MPNs) experience a substantial symptom burden that decreases their quality of life. Little is known about the prevalence of distress and the utilization of SS in pts with myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET). Hence, we designed and present results of a single center study evaluating levels of distress, SS evaluations, and frequency of hospitalizations in pts with MF, PV, and ET.
METHODS Adult pts (age≥18) diagnosed with MF, PV, or ET followed at Levine Cancer Institute (LCI) in Charlotte, NC between 1/1/2017-6/30/21 were included. All included pts were established with a LCI clinician (>1 visit) and had completed an electronic distress survey (EDS), which contained sociodemographic questions and patient-reported outcomes (PROs): distress level, physical symptoms, screening for depression (PHQ-2) and anxiety (GAD-2), daily function, social support, and emotional/spiritual concerns. Pts diagnosed with another hematologic or solid tumor malignancy at the time of survey were excluded. EDS responses, mutations, disease risk assessments, SS visits, ED visits, and hospitalizations were collected retrospectively. Primary objective was to measure the proportion of pts who reported significant distress (score ≥4, based on NCCN guidelines) and were evaluated by ≥1 SS (psychiatry, social work, psychology, chaplaincy, palliative care, and integrative oncology) within 6 months of screening; the proportions with automated referrals based on system triggers (distress score ≥7, GAD-2 ≥5, or PHQ-3 ≥3), unplanned hospitalizations, and ED encounters were also measured. Disease characteristics and PROs were compared between distress groups (score ≥4, 4-6, ≥7) with Fisher's Exact test. Statistical analysis was performed in SAS v9.4; P<0.05 was considered statistically significant. This study was approved by the institutional IRB.
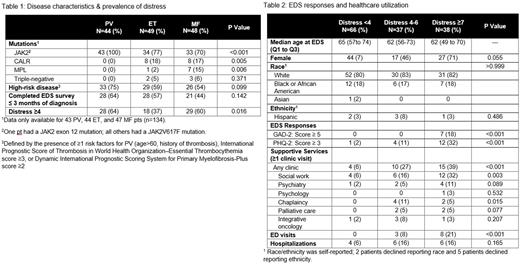
RESULTS Pt characteristics and demographics are presented in Table 1. One hundred forty-one pts were included: PV 44 (31%), ET 49 (35%), MF 48 (34%). Median age was 63 years, and 62% were female. Seventy-five (53%) pts reported significant distress, of which 44 (59%) were ≤3 months of diagnosis. Sixty-four percent of PV pts and 60% of MF pts reported significant distress versus 37% of ET pts (P=0.016).
SS and healthcare utilization is presented in table 2. The proportion of pts expressing significant distress and being evaluated by SS within 6 months of screening was 33% (n=25). Of 38 (27%) pts who met criteria for an automated referral, 15 (39%) were evaluated by SS within 6 months. Distressed pts (DPTS) had more ED visits (15% vs. 0%, P<0.001) and unplanned hospitalizations (16% vs. 6%, P=0.11).
DISCUSSION Despite consensus recommendations, most DPTS were not evaluated by SS. Among DPTS evaluated by SS, social work was most frequently utilized. PV and MF pts were more likely to report distress compared to ET. Limitations to this analysis include lack of data of SS utilization outside of our system, barriers to accessing SS within our system, treatment characteristics, and impact on outcomes. Future work should identify ways to better use patient-reported outcomes to promote early intervention and access to SS.
Disclosures
Symanowski:Eli Lilly & Co.: Consultancy; Camarus: Consultancy; CARsgen: Consultancy; Immatics: Consultancy. Walsh:Helsinn: Consultancy, Other: Unpaid consultancy. Grunwald:Genetech/Roche, Incyte Corporation, Janssen: Research Funding; Medtronic: Current equity holder in private company; Daiichi Sankyo, Gamida Cell, Gilead Sciences, Incyte Corporation, Invitae, Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Sierra Oncology, Stemline Therapeutics: Consultancy; AbbVie, Agios/Servier, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma, Daiichi Sankyo, Gamida Cell, Gilead Sciences, Incyte Corporation, Invitae, Karius, Novartis, Ono Pharmaceuticals, Pfizer, ,: Consultancy. Chojecki:Incyte: Research Funding; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal