Abstract
Introduction: The neurological complications of sickle cell disease (SCD) are particularly devastating and range from "overt stroke" to cerebral small vessel disease (CSVD), including silent cerebral infarction (SCI) and cognitive deficits despite the absence of magnetic resonance imaging (MR)-detectable evidence of cerebral injury. This suggests the possibility of additional, possibly covert, mechanisms of injury. Perivascular (Virchow-Robin) spaces (PVS) are fluid filled spaces that surround cerebral vessels penetrating or leaving the brain parenchyma and become visible when enlarged (dilated), due to inflammation or hypertension. The presence of dilated PVS (dPVS) in the basal ganglia (BG) and centrum semiovale (CO) are rare in children and have been associated with multiple neurodegenerative and neuroinflammatory disorders in aging populations. dPVS in the BG specifically are associated with CSVD, while those in the CO are associated with neuroinflammation. However, until now, the presence of dPVS has not been studied in either adults or children with SCD. Our overall objectives were to use MR images from children who were screened and/or participated in the Silent Cerebral Infarct Treatment in SCD (SIT) trial to 1) determine the burden of dPVS in children with SCD, specifically within the basal ganglia and centrum semiovale, and 2) correlate these findings with other markers of CSVD, specifically silent cerebral infarctions.
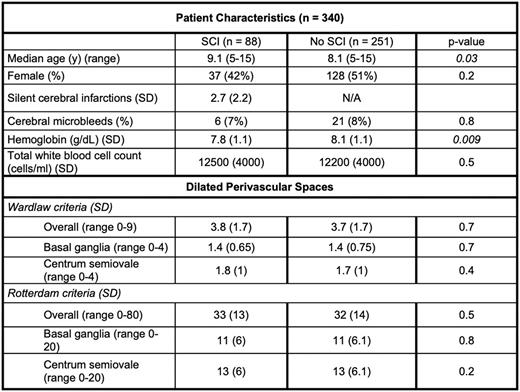
Methods: The SIT trial was a multicenter, randomized clinical trial that evaluated the effect of monthly chronic transfusions to prevent the development of new SCI in children with SCD. As part of the enrollment process, all children underwent an initial MRI. We used both the Wardlaw (currently used as part of the international consensus criteria for evaluating CSVD) and Rotterdam criteria to quantify dPVS burden on T2-weighted screening MR images for 340 participants from the SIT trial. Briefly, both rating scales evaluate dPVS in the BG, CO, and midbrain (MB); the Rotterdam criteria also includes the hippocampus. The Wardlaw criteria categorize dPVS on an ordinal scale from 0 (none) to 4 (> 40) in the BG and CO and 0 or 1 point (absent or present) for the MB, with a total score of 9 as the maximum dPVS burden from all 3 brain regions. The Rotterdam criteria uses a semi-continuous scale and specifies counting dPVS to a total of 20 for each region for a maximum combined dPVD burden score of 80. Additional variables in our analysis were participant's age, hemoglobin, total white blood cell (WBC) count, and presence of SCI at baseline. We categorized dPVS burden score from the Wardlaw rating scale into low (0-2), medium (3-4), and high ( 5) and performed an ordinal multivariate logistic regression with age, sex, presence of SCI, hemoglobin, and total WBC count as independent variables.
Results: We completed the two dPVS rating scales on 340 initial (screening) MR images from 340 children screened for the SIT trial, with a median age of 8.3 years (range 5 - 15 years). Eighty-eight (26%) had evidence of at least one SCI. The mean hemoglobin was 8 ± 1.1 g/dL and total WBC count was 12,000 ± 4200 cells/ml. The mean dPVS burden score from the Wardlaw criteria was 3.7 ± 1.7, while for the Rotterdam criteria, the mean was 32 ± 14 (Table). Global and regional dPVS scores from the two rating scales were well correlated (p < 0.001, r = 0.72 - 0.8). dPVS score in the BG and CSO was moderately correlated (p < 0.001, r = 0.45). There was no significant sex difference in dPVS burden (mean Wardlaw rating: males = 3.9 versus females = 3.6, p = 0.08). Multivariate analysis show that increasing age was associated with dPVS burden (p = 0.005), as was increasing hemoglobin, which showed a trend towards association (p = 0.06), potentially due to increased pressure within microvasculature, resulting in extravasation of fluid into the surrounding space. Surprisingly, presence or size of SCI were not associated with dPVS burden.
Conclusions: Children with SCD have evidence of more than expected dilated perivascular spaces compared to not only healthy children but also adults, which increase with age. Although likely involved in neurological injury, dilated PVS in children with SCD do not appear to be associated with the presence of CSVD phenotypes such as SCI. We reasoned that in SCD, dPVS may be sequelae of underlying neuroinflammation. Future studies are needed to examine correlation of dPVS with biomarkers of neuroinflammation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal