Abstract
Background: Sickle cell disease (SCD) is an inherited red blood cell disorder characterized by chronic anemia and hemolysis, inflammation, and vaso-occlusion that manifests clinically as recurrent, painful vaso-occlusive crises (VOCs) and widespread organ damage. VOCs are the main reason for admission to a healthcare facility (eg, hospital, emergency department [ED], observation unit, or day care). Frequent admission for VOCs is an indicator of severe disease and is associated with early mortality. In the United States, 33% of patients admitted to a healthcare facility for VOCs are readmitted within 30 days, and approximately 50% of patients are readmitted within 90 days. These high readmission rates place an enormous burden on patients, caregivers, and the healthcare system. US readmission rates may not represent 90-day readmission rates among individuals with SCD outside of the United States due to variability in healthcare settings, practices, access, and other potentially confounding factors.
Objective: To explore regional differences in 90-day readmission rates for VOCs, this retrospective chart review was conducted in a sample of patients with SCD at selected sites across geographic regions.
Methods: Medical records from patients with SCD (all genotypes), aged ≥12 years, who were admitted to the ED or hospital for management of a VOC during a 2-year period were evaluated for this retrospective chart review. The evaluation took place at 8 sites on 5 continents (North America, Africa, Asia, Europe, and South America).
Eligible patients had a history of painful VOC episodes, including complicated VOCs of acute chest syndrome, hepatic sequestration, splenic sequestration, and priapism, requiring admission to a medical facility (hospital, ED, observation unit, or day care) for treatment. For each patient, dates of admission for a VOC requiring treatment with parenteral (intravenous or intramuscular) pain medications (eg, nonsteroidal anti-inflammatory drugs, narcotics) in the selected time were collected.
Patients with SCD who had 2 to 10 VOCs within year 1 were eligible for inclusion in the chart analysis. The first admission for VOC during the second year of the study was designated as the index VOC. A subsequent VOC occurring within 90 days of the index VOC was used to determine the 90-day readmission rate.
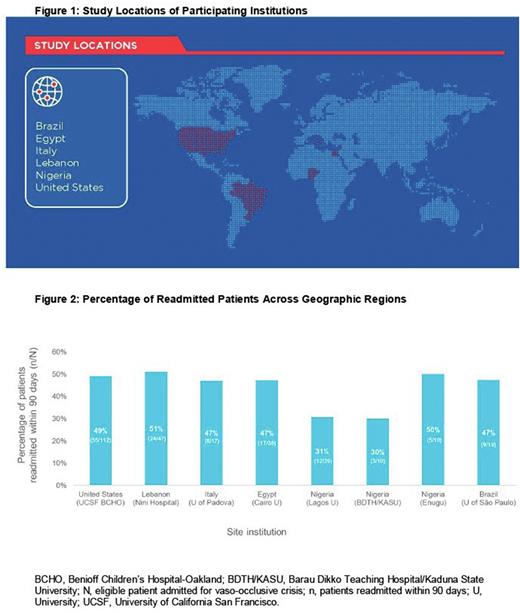
Results: The study included a review of 500 patient charts across 8 geographic regions in 6 countries: Brazil, Egypt, Italy, Lebanon, Nigeria, and the United States (Figure 1). Out of the 500 patient charts reviewed, 290 (58%) were eligible for this study. Of the eligible patients with SCD who were admitted for a VOC, those who were readmitted within 90 days were included for analysis. The proportion of patients readmitted for a VOC within 90 days ranged from 30% to 51% (Figure 2).
Conclusions: Recurrent and prolonged VOC-related hospitalizations substantially disrupt the daily lives and emotional well-being of patients with SCD and are associated with increased risk of mortality. VOCs also remain the main cause for inpatient admissions, and there is an urgent need to reduce the burden of VOC-related hospitalizations. This study provides insights into 90-day readmission rates for VOCs in patients with SCD across different geographic regions. Rates of readmission due to VOCs were generally similar across the sites and consistent with rates observed in the United States. The 90-day readmission rates observed in this study suggest that similar factors may influence VOC hospitalization rates. Identification of these factors is critical for formulating interventions to reduce readmissions and the associated social and economic burden, and for facilitating efficient allocation of resources. Future analyses will include the assessment of site and practice-specific factors contributing to these results to inform physicians treating patients with SCD on potential ways to alleviate this burden on patients, caregivers, and the medical community.
Funding: Global Blood Therapeutics.
Disclosures
Colombatti:AddMedica: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding; Novartis: Consultancy; Bluebird Bio: Consultancy, Research Funding; Novo Nordisk: Consultancy; Forma Therapeutics: Consultancy. Hoppe:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Vichinsky:Agios: Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy. Hamdy:Novartis: Consultancy, Research Funding; Chiesi: Consultancy, Research Funding; Amgen: Consultancy; Bayer: Consultancy; Bristol Myers Squibb: Consultancy; Novo Nordisk: Consultancy; Roche: Consultancy; Takeda: Consultancy. Davis:Global Blood Therapeutics: Current Employment. Bello-Manga:Global Blood Therapeutics: Consultancy. Pinto:Global Blood Therapeutics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Masters Pharmaceutical: Consultancy; Chiesi: Consultancy; Roche: Research Funding. Inati:Octapharma: Research Funding; Vifor Pharma: Research Funding; AstraZeneca: Research Funding; Imara: Research Funding; Agios Pharmaceuticals: Research Funding; Forma Therapeutics: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Novo Nordisk: Consultancy; Roche: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal