Abstract
Background: The clinical characteristics of splanchnic vein thrombosis (SVT) in pediatric patients and its optimal treatment strategies are unknown.
Aim: We aimed to study the risk factors for pediatric SVT and the efficacy and safety of anticoagulant therapy.
Methods: MEDLINE and EMBASE were searched up to December 2021. We only included studies which reported SVT treatment and outcomes, including rates of SVT re-canalization, progression or recurrence, as well as rates of bleeding and mortality.
SVT anticoagulation responsewas pooled using inverse variance and generalized linear mixed and random-effects models.
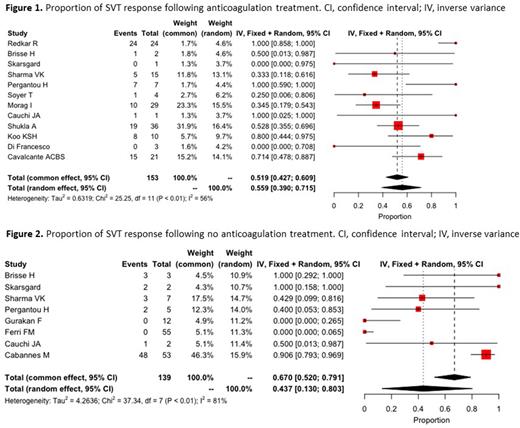
Results: A total of 508 patients were included across 17 studies. Of pediatric SVT cases, 308 presented with portal vein thrombosis, 175 with Budd-Chiari syndrome, 3 with splenic vein thrombosis, 2 with mesenteric vein thrombosis, and 20 with thromboses involving multiple sites. Anticoagulant therapy was prescribed in 187 patients; However, outcome was reported only in 153 of these patients. Of 321 patients who did not receive anticoagulation, outcome was reported in 139. The pooled proportion of response to anticoagulant therapy (assessed by any recanalization) was 55.9% (95% confidence interval (CI): 39%-71%, I2 = 56%) (Figure 1). In patients who did not receive anticoagulation, recanalization rate was 43.7% (95% confidence interval (CI): 13%-80%, I2 = 81%) (Figure 2). Major bleeding was reported in 3.7% (7/187) of patients who received anticoagulant therapy and 0.6 % (2/321) in patients who did not receive anticoagulant therapy, and non-major bleeding was reported in 4.8% (9/187) and 14.3% (46/321) respectively. Mortality was reported in 10.4% (53/508) of pediatric SVT patients (42 were treated with anticoagulation and 11 did not). VTE recurrence was rare, and reported in 0.8% of SVT patients (4/508) all of whom received anticoagulant therapy.
Conclusions: In pediatric patients with SVT, anticoagulant therapy appears to be associated with high recanalization rates and low risk of thrombus progression and bleeding. The incidence of VTE recurrence remains low and comparable to the risk of VTE recurrence in pediatric patients with other types of VTE.
Disclosures
Levy-Mendelovich:Pfizer: Honoraria, Research Funding; NovoNordisk: Research Funding; BSF: Research Funding. Barg:Pfizer, Roche, NovoNordisk: Honoraria. Ageno:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS-Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria; Viatris: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Norgine: Membership on an entity's Board of Directors or advisory committees; Leo Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kenet:Shire: Research Funding; Alnylam: Research Funding; BPL: Consultancy, Research Funding; Opko Biologics: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Roche: Consultancy, Research Funding; UniQure, SPark, Sobi, CSL: Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Sanofi-Genzyme: Consultancy, Honoraria; ASC Therapeutics: Consultancy; Novo Nordisk: Consultancy, Honoraria; BioMarin Pharmaceutical Inc.: Consultancy, Honoraria. Cohen:Pfizer: Research Funding; PlasFree: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal