Abstract
Background: Omacetaxine mepesuccinate (OM), a semi-synthetic form of homoharringtonine (HHT), mechanistically inhibits aminoacyl-tRNA binding to ribosomal acceptor "A” sites, preventing mRNA translation (Wetzler,Curr Pharm Des 2011, 17:59-64), and causing a rapid decline of short-lived proteins e.g., c-myc, cyclin D, the anti-apoptotic proteins cFLIP, survivin and Mcl-1, an important anti-apoptotic regulator in AML.While HHT added to cytarabine and an anthracycline improved CR and 3-yr EFS in de novo AML patients (pts; Jin et al, Lancet Oncology 2013, 7:599-608), meta-analysis of Chinese studies in elderly pts indicated a CR of 47.5%.
Aims: Early analysis of AML-02, a phase Ib trial of escalating doses of OM (an FDA-approved drug) added to a standard 7 + 3 induction regimen was described (NCT02440568; Christian et al., Blood, 2018, 132:5218). Here we report long-term pt outcomes and studies of their correlation with in vitro OM effects on protein translation and apoptosis sensitivity.
Methods: From June 2015 to June 2018, 22 pts aged 18-70 yrs with newly diagnosed AML entered AML-02, receiving escalating doses of OM administered SQ q12h d1-7 with cytarabine (100mg/m2 CIV) d1-7 and Idarubicin 12mg/m2 IV d1-3. Four OM dose levels were tested starting with cohort #1 at 0.625mg/m2 sc q12h (also 1.25, 2.0, and 3.0mg/m2 q12h cohorts). The primary endpoint, the optimally safe and active dose (OD) was determined using a Bayesian adaptive design, EFFTOX, which considers trade-offs between efficacy and toxicity to establish the OD (Shah et al, Leukemia, 2015; 29:1945). Secondary endpoints included OS, RFS and EFS. Residual disease at d14 was deemed a study failure and pts were taken off study. Hematological toxicity (HT) was defined as incomplete hematologic recovery at d49 with the BM free of leukemia. Post-induction therapy consisted of standard high dose cytarabine consolidation chemotherapy or allogeneic HSCT, based on cytogenetic risk (ELN 2010). Potential correlations of the effects of OM on protein translation rates, measured using the OP-Puro technique, and apoptosis sensitivity, measured using BH3 profiling techniques, on pretreatment AML samples and subsequent patient outcomes were also examined. Follow-up is complete to August 1st, 2022.
Results: Median age was 58 yrs, 12 pts had adverse cytogenetics, 6 intermediate and 3 favorable risk (1 unknown due to fibrosis); overall 73% (16) had adverse cytogenetics or secondary AML. Earlier results (Christian et al) indicated no HTs in 21 evaluable pts, and OD was established as 2.0mg/m2.
CR was 45.5%, median OS 22.7 months (OS at 4 yrs 45%), and EFS 80 days. The median OS is now 24.2 months (95% CI 18.3-39.7), median EFS is 80 days (95% CI 280-964), and OS at 6 years remains at 45%. There were no new TEAEs.
Notably, of 10 pts achieving CR, 1 pt relapsed at D60, 1 was lost to follow-up and 1 died of opiate overdose (in CR). Five of the remaining 7 underwent HSCT and all 7 remain in remission. In pts achieving CR, median RFS is 62.4 months (95% CI 42.6-82.2; Fig.1), suggesting potential LSC impact.
Common adverse effects included neutropenia (82%), nausea (64%), vomiting (59%), diarrhea (45%), hyperglycemia (41%), and a transient desquamating skin reaction (27%) that is dose-related and may be due to OM-mediated downregulation of cFLIP levels in sweat glands (Hwang et al, British Journal of Haematology, 2021; 195:e138-e146).
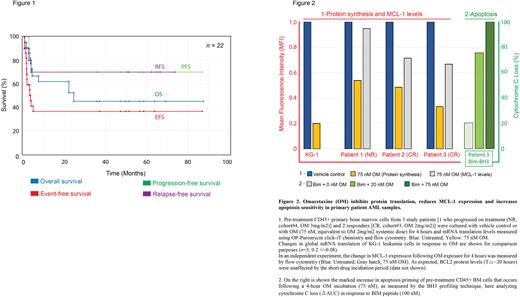
In in vitro studies, 3 pretreatment AML pt BM samples were analyzed after a short-term (non-cytotoxic) OM treatment for 4 hours at a dose (75 nM) equivalent to calculated systemic OM levels achieved in cohort#3 (2mg/m2). Responding pt samples (CR;n=2) had a greater reduction in protein translation than the non-responder (NR). With exposure to OM, CR pts also had greater reductions of proteins with a short T1/2, such as anti-apoptotic protein MCL-1, with a marked increase in cytochrome C loss, indicating high mitochondrial priming and sensitivity to apoptosis (Fig.2).
Conclusion: This small phase Ib study of the addition of OM to a standard 7 + 3 regimen in a diverse population with predominantly high-risk AML and frequent co-morbidities demonstrates a manageable safety profile, acceptable efficacy and resulted in a prolonged DoR. Studies of pretreatment pt samples suggest that analysis of blast protein translation inhibition and especially BH3 profiling responses to OM may predict susceptibility of LSC to Omacetaxine, and potentially patient outcomes.
Disclosures
Patel:Exelixis: Current Employment. Saraf:ORIC: Consultancy; Agios: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding, Speakers Bureau; FORMA Therapeutics: Consultancy, Research Funding. Quigley:Servier: Speakers Bureau; Alnylam: Speakers Bureau; Agios: Speakers Bureau; Rigel: Other: Advisory Board.
OffLabel Disclosure:
Omacetaxine an inhibitor of protein translation synergizes with cytarabine to increase apoptosis in leukemic cells. Its safety and efficacy was explored in this trial in addition to 7+3 regimen
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal