Abstract
Introduction: High-dose methotrexate (HD-MTX) is a key component in treatment of acute lymphoblastic leukemia (ALL) but associates with several toxicities. In addition to myelosuppression and emesis, HD-MTX has specific adverse effects such as oral mucositis, nephrotoxicity, hepatotoxicity, dermatitis, and methotrexate-related stroke like syndrome. Toxicity during prior courses of HD-MTX, delayed MTX elimination and pre-existing nephropathy are associated with toxicity, but information on other risk factors is scarce.
Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL2008 protocol included an intensive consolidation with HD-MTX for non-high risk patients. We aimed to describe the incidence of and risk factors for HD-MTX induced toxicity during consolidation therapy.
Method: This is a retrospective study, including patients aged 2-<18 years with ALL, treated according to the non-high risk arm of the NOPHO ALL2008 protocol in Stockholm and Uppsala, Sweden, during 2008-2019. Data on toxicities during the consolidation was collected after the three HD-MTX courses (5 g/m2) during consolidation. Concomitant chemotherapeutic agents were pegylated asparaginase, vincristine and oral mercaptopurine. The registered toxicities included hematological toxicity (transfusions and/or neutropenia), infections or mucositis requiring hospitalization, administration of intravenous antibiotics or parenteral nutrition, liver toxicity, dermatitis, increased creatinine and CNS toxicity (definitions in Table). Clinical variables included age, immunophenotype, renal ultrasound at diagnosis, blood cell count and creatinine at diagnosis and at start of HD-MTX, BMI standard deviation scores (BMI-SDS), and weight change during induction.
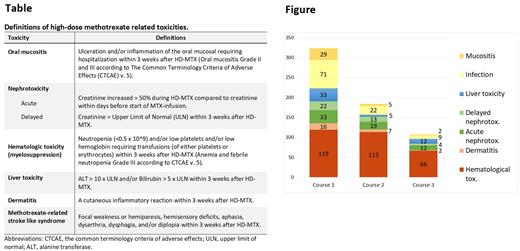
Results: The study cohort comprised 143 patients with 426 high-dose HD-MTX courses in total. The median age at diagnosis was 5.1 years (IQR 5.09). Toxicity was common, especially after the first HD-MTX course (Figure). Every fifth patient was hospitalized due to mucositis after the first HD-MTX and 79% of those needed parenteral nutrition. Infections were frequent; 49.7% were admitted to in-patient care with infection after the first HD-MTX, of whom all but one (70/71) were treated with intravenous antibiotics and 15% had a positive blood culture. Hematological toxicity, whilst most frequent after the first HD-MTX, was common after all three HD-MTX courses.
A significantly higher risk of recurrent toxicity after the first HD-MTX was found for mucositis (Odds ratio (OR) 5.4; 95% confidence interval (CI) 1.1- 26.1; p=0.034), dermatitis (OR 32.8; 95% CI 5.5-197; p=<0.001), delayed nephrotoxicity (OR 59.0; 95% CI 11.2-307; p=<0.001). Liver toxicity and acute nephrotoxicity were not associated with an increased risk of recurrence after subsequent HD-MTX courses.
When risk factors were analyzed separately for the first HD-MTX course, mucositis was more frequent in patients with weight loss (> 5%) during induction (p=0.007). The median BMI-SDS at diagnosis was also lower in patients with mucositis than those without (p=0.081). High MTX concentration (MTX >1 µM at hour 42) at first HD-MTX was associated with an increased need of thrombocyte- (p=0.021) and erythrocyte transfusion (p=0.041).
Mildly (42-hour plasma MTX ≥1.0-3.9 µM), moderately (42-hour plasma MTX ≥4.0-9.9 µM) or severely delayed (42-hour plasma MTX ≥10 µM) elimination was seen in 38.0% (moderate; 5.6%; severe; 1.4%), 17.6% (moderate 2.1%) and 19.9% (moderate; 1.4%; severe; 0.7%) after first, second and third HD-MTX courses, respectively. Higher MTX concentration and slower clearance to non-toxic levels was associated with acute and delayed nephrotoxicity after the first HD-MTX (p=0.001).
Conclusion: Our study demonstrated that severe toxicities that warrant hospitalization are very common, especially after the first HD-MTX. Low BMI at diagnosis and weight loss during induction was related to higher risk for mucositis and high MTX concentration and delayed excretion predicted nephrotoxicity after the first HD-MTX.
Disclosures
Heyman:Swedish Childhood Cancer FundFoundation: Current Employment, Research Funding; NovaLab: Research Funding; Pfizer: Research Funding; Amgen: Consultancy, Research Funding; Servier: Research Funding. Ranta:Roche: Consultancy, Other: Steering committee, compensation paid to Karolinska Hospital.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal