Abstract
Background
Numerous studies convincingly demonstrate that measurable residual disease (MRD) in AML at time of allogeneic cell transplantation (alloHCT) is a powerful, independent predictor of subsequent relapse. Attempts to eradicate AML MRD pre-transplant with consolidation chemotherapy have been unsuccessful, with reported MRD conversion rates as low as 20% (Appelbaum et al., Blood 2019). Tagraxofusp is an IL-3 truncated diphtheria toxin recombinant protein that binds CD123 with high affinity. Preliminary data using tagraxofusp as consolidation therapy in AML patients has demonstrated good tolerability (Lane et al., Blood 2017). Pathways of resistance to tagraxofusp have been identified preclinically as loss of the intracellular target for diphtheria toxin, diphthamide, through methylation of an enzyme involved in diphthamide synthesis. Treatment with a hypomethylating agent has the potential to restore expression of diphthamide and increase tagraxofusp sensitivity in AML cells (Togami et al., J Clin Invest 2019).
Design
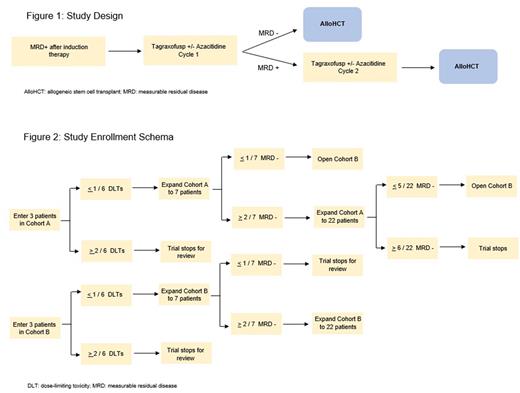
This is a single-arm, open-label, multi-center phase Ib/II clinical trial to evaluate the safety and effectiveness of tagraxofusp +/- azacitidine in AML patients who are in first or second remission with detectable MRD and are planned to undergo alloHCT. MRD will be measured by multi-dimensional flow cytometry with sensitivity of 0.02% (Hematologics). Patients will be enrolled in a modified 3+3 dose escalation design with two cohorts: cohort A) tagraxofusp 12 mcg/kg IV qday on days 1-5; and cohort B) azacitidine 75 mg/m2 subq qday on days 1-5 plus tagraxofusp 12 mcg/kg qday on days 8-12. Patients achieving MRD-negativity after cycle 1 will proceed to alloHCT. If a patient remains MRD-positive, an additional cycle of investigational therapy will be administered. All patients will proceed to alloHCT after no more than 2 cycles of investigational therapy. Both cohorts contain a dose escalation and a dose expansion phase. Cohort B will open if cohort A is not efficacious. Up to 44 participants will be enrolled in this trial at University of California Los Angeles and University of California San Francisco (two constituent sites of the University of California Hematologic Malignancies Consortium).
Objectives
The primary objective is to estimate the rate of conversion from MRD-positive pre-investigational therapy to MRD-negative after 1 or 2 cycles of investigational therapy. Key secondary objectives include to determine the safety of tagraxofusp +/- azacitidine in this patient population and to evaluate if adverse effects of this investigational therapy lead to delays in alloHCT. Exploratory objectives will involve use of single cell RNA sequencing to investigate if response to investigational therapy is predicted by leukemia-specific biomarkers and to evaluate if putative cell surface markers (inferred from RNA detection) are associated with MRD persistence.
Main Outcomes
The primary endpoint is MRD status after 1 or 2 cycles of investigational therapy. Key secondary endpoints include dose-limiting toxicities (any grade 4 or 5 non-hematologic toxicity or any grade 3 or greater hematologic toxicity at day 42) and number of days between investigational therapy and alloHCT.
Conclusions
There is an unmet need to improve outcomes in AML with detectable MRD pre-transplant. The strategy in this study is to employ tagraxofusp as an alternative mechanism of action to eradicate chemo-resistant AML cells responsible for subsequent relapse. In order to mitigate any potential resistance the residual leukemia population may have against tagraxofusp, azacitidine will be added to optimize the likelihood for MRD negativity and the possibility for improved outcomes after alloHCT. This study was sponsored by the ASH CRTI.
References
Appelbaum J, Loeb A, Gardner K, et al. Additional Cytotoxic Chemotherapy Is Unlikely to Eliminate Measurable Residual Acute Myeloid Leukemia (AML). Blood. 2019;134(Supplement_1):260-260.
Lane AA, Sweet KL, Wang ES, et al. Results from Ongoing Phase 1/2 Trial of SL-401 As Consolidation Therapy in Patients with Acute Myeloid Leukemia (AML) in Remission with Minimal Residual Disease (MRD). Blood. 2017;130(Supplement 1):2583-2583.
Togami K, Pastika T, Stephansky J, et al. DNA methyltransferase inhibition overcomes diphthamide pathway deficiencies underlying CD123-targeted treatment resistance. J Clin Invest. 2019;129(11):5005-5019.
Disclosures
Oliai:Arog: Research Funding; Orca Bio: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Seagen: Research Funding. Logan:Amgen: Research Funding; Amphivena: Research Funding; Astellas: Research Funding; Autolus: Research Funding; Jazz: Research Funding; Kadmon: Research Funding; Kite/Gilead: Research Funding; BMS: Consultancy; Pfizer: Consultancy; Pharmacyclics: Research Funding; Abbvie: Consultancy. Schiller:Cellerant: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Regimmune: Research Funding; AbbVie: Research Funding, Speakers Bureau; Actinium: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Jazz: Consultancy; Stemline: Speakers Bureau; Mateon: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Deltafly: Research Funding; FujiFilm: Research Funding; Glycomimetics: Research Funding; Arog: Research Funding; AVM Biopharma: Research Funding; Onconova: Research Funding; Stemline: Research Funding; Constellation: Research Funding; Actuate: Research Funding; Forma: Research Funding; CTI: Research Funding; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; Pfizer: Research Funding; Janssen: Research Funding; Gamida: Research Funding; Cellectis: Research Funding; Millennium: Research Funding; Medimmune: Research Funding; Agios: Consultancy, Honoraria; AltruBio: Research Funding; Samus: Research Funding; Gilead: Research Funding; Trovagen: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; Geron: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Deciphera: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Ono Pharma: Honoraria; Sellas: Research Funding; PreCOG LLC: Research Funding; Genentech-Roche: Research Funding; AstraZeneca: Honoraria; Astellas: Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding.
OffLabel Disclosure:
Tagraxofusp is approved for the treatment of blastic plasmacytoid dendritic cell neoplasm in adults and in pediatric patients 2 years and older.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal