Abstract
Introduction: Mutations in TP53 confer an adverse prognosis in patients (pts) with AML and HR-MDS. Comprehensive analyses of the genetic, epigenetic, and immunologic landscape of TP53-M AML and HR-MDS are lacking, especially associations of allelic status of TP53 with immune and epigenetic disease phenotype.
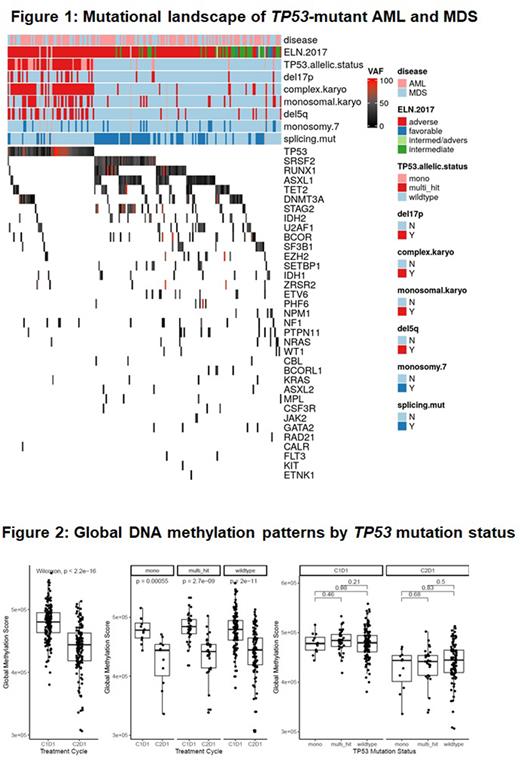
Methods: Using a large, randomized study with extensive and prospective clinical, histopathological, cytogenetic, molecular, epigenetic, and immunologic annotations, we compared data from 61 TP53-M (37 AML, 24 HR-MDS) and 144 TP53 wild-type (TP53-WT; 89 AML, 55 HR-MDS) pts. Pts were randomized to azacitidine (AZA) monotherapy or AZA + anti-PDL1 antibody durvalumab, which did not improve clinical outcomes (Zeidan et al. Blood Advances 2022). A 38 gene-targeted myeloid mutation analysis from screening bone marrow (BM) was performed at Munich Leukemia Laboratory. Only pathogenic TP53 variants with variant allele frequency (VAF) ≥2% were included. Pts with a common SNP or VUS in the TP53 gene were classified as TP53-WT. Multi-hit TP53 mutations were defined as previously published by Grob et al. (Blood 2022) which confirmed that TP53-M AML and HR-MDS can be grouped as one entity.
BM aspirates from screening and cycle 3 day 22 (C3D22) were used to study gene expression profiles by RNA-sequencing and immune phenotyping by flow cytometry. Gene set enrichment analysis (GSEA) using the MSigDB and H (Hallmark) gene sets as provided by the msigdbr R package were performed. Flow cytometry data were analyzed into a collection of 170 reportable subpopulations in the immune cell and immune checkpoint panel and 68 reportables in the tumor/blast panel. DNA methylation of peripheral blood samples was assessed using Illumina's Infinium Human Methylation EPIC methylation array pre-treatment and at C2D1. Global DNA methylation scores (GDMS) and focal DNA demethylation in PD-1, PD-L1 and PD-L2 regulatory regions were analyzed.
Results: Of the 61 TP53-M and 144 TP53-WT pts, 85.4% and 10.4% had complex karyotype, respectively. TP53-M pts had fewer co-occurring mutations compared with TP53-WT (mean number of mutations: 1.8 [SD: 0.77] vs 2.9 [SD: 1.4]; P <0.001). Distinct mutational patterns by TP53 mutational status were noted (Figure 1). The median VAF for TP53 mutations was 40%, and 90% had a VAF ≥10%. 22 (11.0%) and 41 (20.5%) pts were mono-hit and multi-hit, respectively.
Global DNA methylation was independent of TP53 mutation status at baseline when comparing mono-hit, multi-hit and wild-type. AZA therapy led to statistically significant decrease in GDMS independent of TP53 mutation status (Figure 2). Various loci of PD-1, PD-L1 and PD-L2 genes showed differences in methylation status by TP53 mutation status, which were reversed on treatment. However, methylation of 2 PD-L2 gene loci (cg07211259 and cg11299543) remained significantly lower for multi-hit TP53-M compared to TP53-W at C2D1.
Patterns of differentially expressed genes were similar for multi-hit TP53-M vs wild-type and for all TP53-M vs wild-type. The number of differentially expressed genes was higher in the AML cohort compared to MDS pts both at baseline (1887 vs 78) and after C3 (116 vs 0) with TP53-M samples having more differentially expressed genes. ZNF560 was the gene with the strongest differential expression by TP53 mutation status. TP53-M pts had higher expression of T-cell genes (e.g., IL7R), markers of proliferation (MKI67), and PD-L1 (CD274) expression compared to TP53-W at baseline. Genes in hallmark clusters of heme metabolism, inflammatory response, cell cycle regulation, and TGF-β signaling pathways were enriched in TP53-M pts in GSEA.
TP53-M pts had higher median levels of PD-L1-positive progenitors (5.6% vs 2.4%; p=0.006) and less myeloid progenitor cells (58% vs 75%; p=0.005) than TP53-WT at baseline. However, there was no shift towards a more exhausted T-cell phenotype among TP53-M pts (CD3+CD4+ PD1+ TIM3+ T-cells and CD3+CD8+PD1+TIM3+ T-cells as % of CD3+CD4+ T-cells and CD3+CD8+ T-cells, respectively).
Conclusion: Although an increased population of cytotoxic T-cells suggest an activated immune system, we also found the upregulation of inhibitory immune checkpoints such as PD-L1 in TP53-mut pts suggesting an immunosuppressive microenvironment as a potential contributor to the poor prognosis of TP53-M AML and MDS with similar findings across disease type (HR-MDS vs AML) and mono- vs multi-hit TP53 mutation status.
Disclosures
Hasle:Bristol Myers Squibb: Current Employment. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Thompson:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Lopes de Menezes:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Rose:Bristol Myers Squibb: Current Employment. Boss:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Halene:Forma Therapeutics: Consultancy. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Fox:Bristol Myers Squibb: Current Employment. Zeidan:Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal