Abstract
Introduction: Fitness and subsets of T lymphocytes collected at the time of apheresis were shown to influence chimeric antigen receptor (CAR) T cell in vivo expansion and thus anti-tumor activity in preclinical and clinical studies. However, whether pre-manufacturing T-cell characteristics significantly affect the outcome of B-cell lymphoma patients (pts) treated with commercial anti-CD19 CAR T-cell therapies, has yet to be completely defined.
Aim: Aim was to evaluate whether leukapheresis phenotypic T-cell features have an impact on post-infusion tisagenlecleucel (Tisa-cel) and axicabtagene ciloleucel (Axi-cel) in vivo expansion and clinical efficacy.
Methods: The T-cell phenotype of apheresis products prospectively collected from 55 pts receiving either Tisa-cel or Axi-cel was characterized at the protein and mRNA level. For flow cytometry (FCM), T-cells were analysed with CD45, CD3, CD4, CD8, CD45RO, CD62L, CD197 and CD95 antibodies and circulating CAR T cells with the CD19-CAR detection reagent (Miltenyi) on a BD FACSCanto II (BD Biosciences) and FlowJo software. RNA of sorted CD3+ cells was digitally quantified using the nCounter 780-gene CAR-T Characterization Panel (NanoString). Disease response was assessed according to Lugano criteria. Cox proportional-hazards model was used to test the association between covariates and progression free survival (PFS), and the globaltest package for R to test the association between PFS and gene expression levels, regarded as continuous variables (under 100.000-permutation condition). The predictive power of the model was tested using Linear Discriminant Analysis for classification of multivariate observations, with leave-one-out procedure.
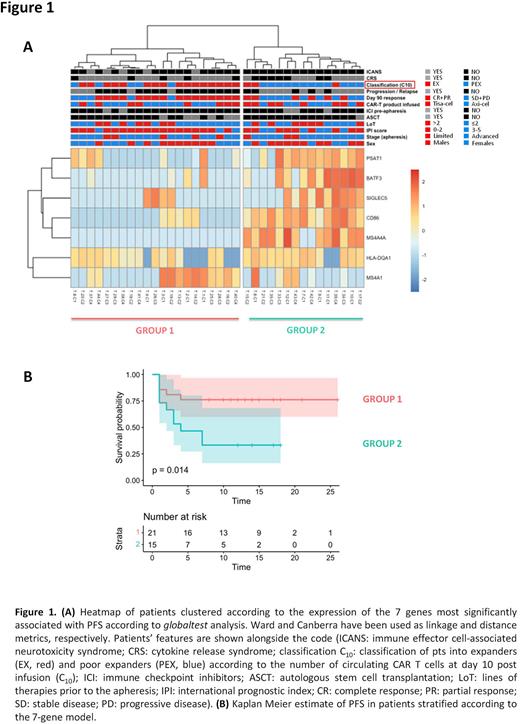
Results: The presence of various T-cell differentiation subsets in apheresis products was assessed (n=55) by FCM. No differences in the frequencies of T naïve [TN (CD45RO−/CD197+/CD62L+)], T stem cell memory [TSCM(CD45RO-/CD197+/CD62L+/CD95+)], T central memory [TCM(CD45RO+/CD197+)], T effector memory [TEM(CD45RO+/CD197-)] and T effector [TE(CD45RO-/CD197-)] subsets among CD4+ and CD8+ T-cells were observed when pts were stratified according to sex (62% were males), age (median age 57, range 26-69), number of prior treatments (median n° of therapies 2, range 2-6), histologies (DLBCL n=40, tFL n=6, PMBCL n=9) and the CAR T-cell product (Tisa-cel n=22, Axi-cel n=33). However, when the median concentration of CAR T cells at day 10 (C10=26 cells/ul) after infusion was used to dichotomize pts into "expanders" (EX, n=28) and "poor-expanders" (PEX, n=27), EX had significantly higher levels of CD3+ and CD8+TSCM cells than PEX (median CD3+: 29,6% vs 20,0%, p= 0.04, median CD8+TSCM: 0.57% vs 0.28%, p=0.02). This is relevant as CAR T-cell kinetics predicted response and survival: EX had in fact more chances to be responders at day 90 (OR: 4.486, 95%CI: 1.447-14.02, p=0.01) and were characterized by longer PFS when compared to PEX (median PFS not reached for EX and 3.2 months for PEX, p=0.02). We then characterized the expression profile of genes included in nCounter CAR-T Panel in apheresed CD3+ selected T-cells of 36 pts (19 EX and 17 PEX) receiving Tisa-cel (n=16) and Axi-cel (n=20). To discover predictors of CAR T-cell expansion and PFS, we identified a 7-gene model (including PSAT1, BATF3, SIGLEC5, CD86, MS4A4A, HLA-DQA1 and MS4A1) capable of distinguishing EX and PEX (Fig 1A) and segregating pts with different survival probabilities (Fig 1B). The predictive power of the 7-gene model was confirmed by leave-one-out cross validation, gaining >95% overall accuracy and only 2/36 misclassified samples. Multivariate analysis showed that the 7-gene model retained significance (p=0.0002) and was negatively associated with PFS along with the lines of therapy and the IPI score as opposed to CAR T-cell expansion.
Conclusions: Our study highlights that unmanipulated leukapheresis share peculiar immunophenotypic and transcriptional features that correlate with CAR T-cell expansion and survival of pts treated with Tisa-cel and Axi-cel. Despite these results warrant functional validation and confirmation in larger cohort (ongoing), the gene signature identified may represent a pre-manufacturing predictive biomarker of CAR T-cell efficacy that might preferentially drive their employment versus other newly approved therapies, such as bispecific T-cell engagers, at the single patient level.
Disclosures
Corradini:Abbvie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Consultancy; AbbVie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano, Novartis, Roche, Sanofi, Takeda: Honoraria; Abbvie, Amgen, Bristol Myers Squibb, Celgene, Gilead/Kite, Janssen, Novartis, Roche, Takeda: Other: Support for attending meetings or travel; Abbvie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GlaxoSmithKline, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Other: Data monitoring board or advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal