Abstract
Introduction: Marginal zone lymphomas (MZL) represent 6-9% of non-Hodgkin lymphomas and can be subclassified into extranodal MZL (EMZL), nodal MZL (NMZL) and splenic MZL (SMZL). Although the majority of MZL cases are characterized by an indolent clinical course, 4-15% of patients experience histological transformation, most commonly into diffuse large B-cell lymphoma (DLBCL). Risk stratification of transformed MZL (tMZL) remains difficult given the paucity of data. The aim of this study was to identify characteristics associated with outcome of tMZL in a large nationwide population-based study.
Methods: Patients with MZL aged ≥18 years, diagnosed between 2014 and 2018 were identified from the Netherlands Cancer Registry (NCR). Transformations to DLBCL occurring between 2014 and 2020 among the MZL patients were identified by cross-linkage within the NCR, resulting in a follow-up of >2 years for each patient. Data on patient characteristics at time of MZL diagnosis and transformation were collected. Risk factors associated with transformation and overall survival (OS) were analyzed using competing-risk analyses with death of any cause as competing risk and Cox regression analyses, respectively. The following variables at MZL diagnosis (1) and at histological transformation (2) were included in the univariable analyses for risk factors associated with transformation (1) and OS after transformation (2): age, gender, MZL subtype, Ann Arbor Stage, LDH, >2 extranodal sites and International Prognostic Index (IPI) score. In addition, time to transformation < 2 years, prior treatment yes/no and R-CHOP versus other systemic treatment regimens were included in the univariable analysis for OS after transformation. Multivariable models were derived using backwards stepwise regression among variables with a p<0.25 in the univariable analysis to enter and p<0.05 to stay in the model.
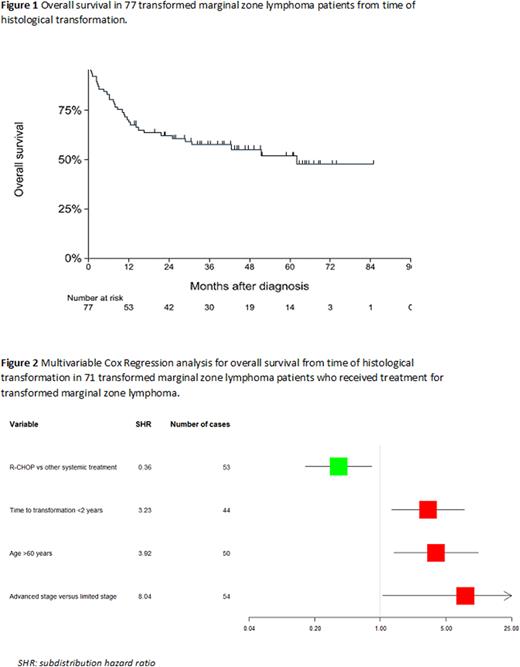
Results: In total, 1830 patients with MZL were identified including 1089 (59.5%) EMZL, 481 (26.3%) NMZL and 260 (14.2%) SMZL. In 1774/1930 (96.9%) cases, MZL diagnosis was histologically confirmed. In the other cases, cytology was used to confirm MZL diagnosis. Among the 1830 MZL patients, 1287 (70.3%) were aged >60 years, 899 (49.1%) had a limited Ann Arbor stage and 1280 (69.9%) had an IPI-score ≤2. The 5-year OS of the whole cohort was 78% (95%CI 0.76 - 0.80). Transformation to DLBCL was observed in 77 patients (4,2%). Transformation was most frequently observed in NMZL in 33/481 (6.8%), followed by 13/260 (5.0%) SMZL, and 31/1,089 (2.8%) EMZL (p<0.01). Median time to transformation was 1.6 years (range 0.25 - 6.0 years). The estimated annual incidence rate (IR) of histological transformation was 11.01 events per 1000 patients per year (95% CI 8.81 - 13.77). No significant difference was observed in the IR between the first year (IR 13.05) and second year (IR 14.54) of follow-up. Considering the entire MZL cohort, elevated LDH at diagnosis of MZL and NMZL subtype were associated with risk of transformation (subdistribution hazard ratio (SHR) 2.29, p<0.01 and SHR 2.08, p<0.01, respectively). The median OS from the time of histological transformation was 62 months (Figure 1). In 6 out of 77 tMZL patients, no treatment was initiated at transformation. In a multivariable analysis including the 71 patients who received treatment for tMZL, age >60 years (hazard ratio (HR) 3.92, p<0.01), time to transformation <2 years (HR 3.23, p=0.01) and Ann Arbor stage III-IV at transformation (HR 8.04, p=0.04) were significantly associated with a lower OS. R-CHOP versus other systemic treatment regimens (e.g. R-DHAP, BR, R-PECC, R-CVP) was associated with a higher OS (HR 0.36, p=0.02) (Figure 2).
Conclusion: In this population-based study, histological transformation was observed in 4.2% of patients with MZL. Outcome was poor, especially for patients aged >60 years and with an advanced stage. Thus, there is a need for a better understanding of the molecular events underlying histological transformation in these patients to optimize and develop new treatment strategies.
Disclosures
Tonino:Roche: Honoraria; Incyte: Honoraria; Takeda: Honoraria. Chamuleau:BMS/Celgene: Honoraria, Research Funding; Gilead: Research Funding; Novartis: Honoraria; Genmab: Research Funding; Abbvie: Honoraria; Roche: Honoraria. Diepstra:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nijland:Roche: Research Funding; Takeda: Research Funding; Genmab: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal