Abstract
Introduction
T-cell large granular lymphocytic leukemia (T-LGL) is a chronic incurable malignancy where once refractory to immunosuppressive therapy, few treatment options and no optimal treatment consensus exists. Alemtuzumab, 10 milligrams (mg) intravenously (IV) administered daily for a total of 10 doses, has been shown to be effective for relapsed or refractory (R/R) T-LGL (Dumitriu, et al. Lancet Haematol 2016); however, it can be associated with severe infusion reactions and infections. Given the palliative nature of T-LGL-directed treatments and the increasing potential for toxicity with cumulative alemtuzumab exposure, we conducted a retrospective analysis to describe the efficacy and safety of abbreviated alemtuzumab regimens in R/R T-LGL patients.
Methods
We identified patients ≥18 years with R/R T-LGL who were treated with short-duration (defined as ≤ 5 doses) alemtuzumab between 2012 and 2022 from the University of Washington lymphoma database. Patients who received prior alemtuzumab were excluded. The primary endpoint was overall hematologic response rate (ORR). Secondary efficacy endpoints included hematologic response rate at 3 and 6 months, rate of transfusion independence, progression-free and overall survival. Safety endpoints included rate of infusion reactions and infections, specifically Epstein-Barr virus (EBV) and cytomegalovirus (CMV) reactivation.
Results
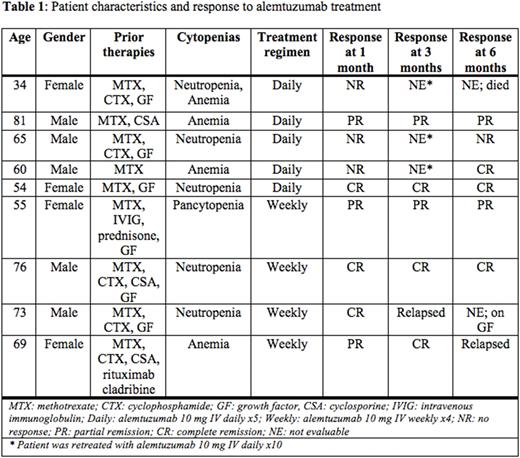
Nine patients meeting criteria were identified (Table 1). Five patients received alemtuzumab 10 mg IV daily for 5 doses and 4 patients received alemtuzumab 10 mg IV weekly for 4 doses. Median age was 65 (range 34-81) years and the median number of prior regimens was 3 (range 1-5). All (100%) patients were refractory to immunosuppressive therapy. Seven (78%), 1 (11%) and 1 (11%) patients had unilineage, bilineage and trilineage cytopenias, respectively. Five patients (56%) had autoimmune comorbidities and five (56%) required periodic transfusions. No patients had myelodysplasia on bone marrow biopsy. Overall, 6 (67%) patients had a hematologic response with 2 patients achieving a partial response (PR) and 4 patients achieving a complete response (CR). ORR at 3 and 6 months was 56% and 44%, respectively. Two (40%) patients achieved transfusion independence. The 3 patients without a hematologic response were retreated with alemtuzumab 10 mg IV daily for 10 doses with 1 subsequently achieving a CR. At a median follow-up time of 7.2 (range 2.3-36.6) months, 3 out of 6 patients who achieved a response had disease relapse; 8 (89%) patients were alive and 1 had died from COVID-19 pneumonia. Among the 5 patients who received alemtuzumab at 10 mg daily for 5 doses, ORR was 40% with 1 patient achieving PR and 1 patient achieving CR. ORR at 3 and 6 months was 40% and 40%, respectively. One (20%) patient had an infusion reaction. Two patients (40%) had subclinical CMV viremia which resolved with valganciclovir. No cases of EBV viremia were noted. Four patients completed alemtuzumab at 10 mg weekly for 4 doses; ORR was 100% with 1 patient achieving PR and 3 achieving CR. Hematologic response rate at 3 and 6 months was 75% and 50%, respectively. Three (75%) patients relapsed at 3, 4 and 14.2 months, respectively; 2 were retreated 4-6 times with alemtuzumab 10 mg IV weekly for 2 to 6 doses each time, with both achieving hematologic responses with each course of treatment. One (25%) patient experienced an infusion reaction. No cases of CMV or EBV viremia were noted.
Conclusions
Short-duration alemtuzumab is effective and well tolerated in R/R T-LGL with responses observed in 67% overall and no cases of CMV viremia detected in the weekly dosing group. Alemtuzumab given at 10 mg IV weekly for 4 doses or until a response is noted followed by retreatment upon relapse was particularly effective; this abbreviated, response-adapted alemtuzumab strategy should be prospectively explored in this patient population.
Disclosures
Poh:Incyte Morphosys: Consultancy, Research Funding. Shustov:Seagen: Current Employment, Current equity holder in publicly-traded company. Gopal:Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy, Honoraria; Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding. Smith:MorphoSys: Research Funding; Nanjing Pharmaceuticals Co., Ltd.: Research Funding; Numab Therapeutics AG: Consultancy; Portola Pharmaceuticals: Research Funding; Abbvie: Consultancy; Viracta Therapeutics: Research Funding; Merck Sharp and Dohme Corp.: Research Funding; Kymera Therapeutics: Research Funding; KITE pharma: Consultancy; Karyopharm: Consultancy; Incyte Corporation: Consultancy, Research Funding; Genentech: Research Funding; Epizyme: Consultancy; Enterome: Research Funding; De Novo Biopharma: Research Funding; Beigene: Consultancy, Research Funding; Bayer: Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal