Abstract
Background- A lower dose of Dasatinib has been shown to have excellent results in CML in CP, even better than standard doses in a single-center study using the innovator drug (Naqvi et al, Cancer, January 2020). We conducted a multicenter trial using a lower dose of generic Dasatinib (Invista, Dr. Reddy's, Hyderabad, India) to see the outcomes.
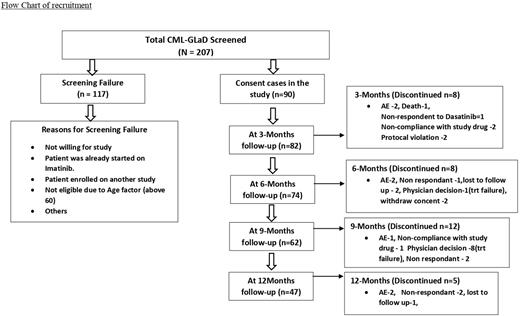
Methods- This is a phase II multi-center investigator initiated study conducted between October 2020 till September 2021. Patients were enrolled from 9 centers across India. The inclusion criteria were CML in CP, aged 18-60 years without any co-morbidities and another cancer. They were enrolled in the trial after a written informed consent. Initial assessment included complete blood counts, bone marrow examination including karyotype, FISH for t (9;22) and serum chemistries. They were given 50 mg daily of generic Dasatinib. Blood counts were monitored once in 1-2 weeks for the first 2 months and monthly thereafter. Dasatinib drug levels and RQPCR was monitored at 3-, 6- and 12-month intervals. The response was defined as per ELN 2020 criteria. The main outcome measure was CCyR (or equivalent molecular response </=1%) at 12 months, the secondary outcome measures included MMR and EMR. Patients who did not tolerate or progressed while on Dasatinib were switched to Imatinib or one of the other TKIs while those who failed at 6 months were switched to standard dose Dasatinib.
Results- Out of the 207 patients who were screened, 90 patients were enrolled into the trial of which 70 (77.8%) were men. The median age was 36.5 years (29-50). The distribution of patients as per Sokal score and ELTS were 35 (39.3%) for high, 44(49.4%)/37(41.6%) for intermediate and 10 (11.2%)/17(19.1%) for low risk respectively.
With a median follow-up of 11(6,12) months, 80 (88.8%), 71 (78.8%) and 39 (43.3%) patients completed 3,6 and 12 months of therapy and the results are available currently. Optimal response at 3, 6 and 12 months as per ELN 2020 criteria was achieved by 44/80 (55%), 42/71 (59.1%) and 19/39 (48.7%) patients.
EMR at 3 months was achieved by 44/80 (55 %) patients. CCyR was achieved by 42/71 (59.1%) at 6 months and 35/39 (89.7%) at 12 months. MMR was achieved by 17/71 (23.9%) and 19/39 (48.7%) patients respectively.
A total of 53 patients developed at least one adverse event. A total of 199 adverse events were recorded, out of which only 13 (6.5%) were of grade ¾. The most common toxicity events were hematological (14), gastrointestinal (55), headache (17) and cough (11). The most common grade ¾ toxicities were hematological (8 events). There were 4 serious AEs during the trial-hematological (2), Ileus (1) and disseminated varicella (1). Toxicities led to a dose reduction in 2 patients and interruption in 18 patients.
Discussion-This is the first multicenter investigator initiated clinical trial involving nine centers from India on Chronic myeloid Leukemia. This reflects the challenges involved in conducting multicenter study like recruitment, patient compliance and default. The CCyR of 89.7% in patients who have completed 12 months of therapy appears promising. The toxicity is comparable to previous studies. The higher proportion of high and intermediate risk patients in this cohort could probably account for the less than expected results compared to previously published data with low dose Dasatinib.
Conclusion-At this interim analysis Dasatinib 50 mg appears to be promising with respect to responses but with a caution considering the fact that the majority of patients had a higher disease load. We need to wait for the final results before Dasatinib 50 mg can be considered as standard of care.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal