Abstract
Background: The therapeutic landscape for CLL/SLL has rapidly evolved with the introduction of novel agents such as Ibr. The informCLL registry (PCYC-1134; NCT02582879) is the largest US-based prospective, observational registry of patients (pts) initiating FDA-approved treatment for CLL/SLL in the era after approval of Ibr. Here, we report real-world (RW) outcomes in pts with CLL/SLL receiving first-line (1L) therapy, including comparison of 1L Ibr versus CIT, from the final analysis of informCLL.
Methods: Pts with CLL/SLL who initiated FDA-approved treatment (within ±45 days of enrollment) were enrolled between 10/2015 and 6/2019. Pts were primarily enrolled from community-based practices (comprising 93% of sites). Data were collected for baseline characteristics, treatment patterns, and safety. Time to next treatment (TTNT) was estimated by Kaplan-Meier method and for comparison of outcomes with 1L Ibr versus CIT, by Cox proportional hazards regression analysis in weighted cohorts. Inverse probability of treatment weighting (IPTW) was performed to balance the baseline characteristics between cohorts. Adverse events (AEs) were evaluated using overall incidence and exposure-adjusted incidence rates (EAIRs).
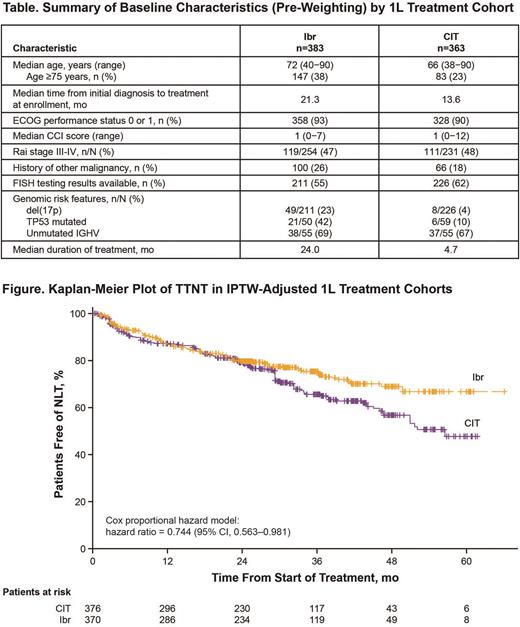
Results: Of the 1459 eligible pts enrolled, 854 were treated in the 1L setting (59%). In the overall 1L cohort, median age was 70 years (≥75 years in 33%); 90% had ECOG performance status of 0 or 1; 47% had Rai stage III/IV (among n=547 with Rai staging); median Charlson Comorbidity Index (CCI) score was 1 (range 0-12) with CCI ≥3 in 16%; median time from initial diagnosis to 1L treatment was 19 mo. FISH testing data were available in 57% of pts in the 1L cohort; among these pts, 12% had del(17p). The most common 1L therapies were Ibr (45%; single-agent Ibr in 43% of all 1L therapies) and CIT (43%: most commonly BR, 24%); immunotherapy was given in 11% while chemotherapy (0.9%) and other novel agents (0.7%) were rare. With a median follow-up of 31.8 mo in the 1L cohort, median TTNT was not reached (NR); estimated proportions of pts without next-line therapy (NLT) were 79% at 24 mo, 71% at 36 mo, and 64% at 48 mo. The 1L cohort included 383 pts treated with Ibr and 363 pts treated with CIT; median follow-up was 31.4 mo for the Ibr cohort and 34.3 mo for CIT. Compared with the CIT cohort, pts in the Ibr cohort were older, had longer time from diagnosis to treatment, were more likely to have a history of other malignancy, and were more likely to have del(17p) or TP53 mutation (Table). Baseline characteristics in the weighted cohorts were balanced. In the IPTW-adjusted cohorts, median TTNT was NR for Ibr and 56.5 mo for CIT; TTNT was improved with Ibr relative to CIT (hazard ratio 0.744; 95% CI, 0.563-0.981) (Figure). Estimated proportions free of NLT in the weighted Ibr cohort were 75% at 36 mo and 69% at 48 mo; rates in the weighted CIT cohort were 66% and 57%, respectively. Median duration of index treatment was 24.0 mo for 1L Ibr and 4.7 mo for CIT. In the Ibr cohort (n=383), serious AEs occurred in 144 pts (38%), most commonly (≥3% of pts) pneumonia (n=23; 6%) and atrial fibrillation (Afib; n=21; 5%); AEs led to discontinuation of Ibr in 135 pts (35%), most commonly Afib (n=19; 5%) and fatigue (n=12; 3%). In the CIT cohort (n=363), serious AEs occurred in 85 pts (23%), most commonly febrile neutropenia (n=13; 4%) and pneumonia (n=11; 3%). AEs led to discontinuation of CIT in 61 pts (17%), and no single AE term led to discontinuation in ≥3% of pts (most common was anemia, 2%). EAIRs per 100 pt-mo for serious AEs were 1.93 with Ibr and 4.55 with CIT. EAIRs per 100 pt-mo for AEs leading to discontinuation were 1.53 with Ibr and 3.09 with CIT.
Conclusion: In this large RW registry of pts with CLL/SLL, results from IPTW-adjusted analysis showed that 1L Ibr was associated with longer TTNT than CIT, with sustained benefit up to 4 years of follow-up. Pts receiving 1L Ibr therapy tended to be older, more often had a history of other malignancy, and more often had high-risk genomic features (del(17p) or TP53 mutation among those tested) than pts treated with CIT. The spectrum and frequency of AEs observed with these 1L regimens appear consistent with data from clinical studies and other RW studies. When adjusted for exposure, rates of AEs leading to discontinuation and serious AEs were lower with Ibr than CIT. Future analyses examining the impact of factors such as age/comorbidity and race are warranted to further our understanding of RW outcomes in pts with CLL/SLL.
Disclosures
Ghosh:Genmab: Consultancy; Loxo Oncology: Consultancy; Novartis: Consultancy; Roche/Genentech: Consultancy, Research Funding; Karyopharm: Consultancy; Incyte: Consultancy; Beigene: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; TG Therapeautics: Consultancy, Research Funding; Seagen: Consultancy; Levine Cancer Institute: Current Employment, Honoraria, Research Funding; Adaptive Biotech: Consultancy; ADC Therapeautics: Consultancy; Epizyme: Speakers Bureau; Morphosys: Research Funding; AbbVie: Research Funding. Sharman:Lilly: Consultancy, Honoraria, Research Funding; Merck: Consultancy; BMS: Consultancy, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Research Funding; Genentech: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Barrientos:MEI: Consultancy; Beigene: Consultancy; Janssen: Honoraria; AstraZeneca: Consultancy, Research Funding; Velosbio/Merck: Research Funding; Oncternal: Research Funding; Pharmacyclics/Abbvie: Consultancy; AbbVie: Consultancy. Brander:DTRM: Research Funding; Pfizer: Consultancy; Ascentage (transitioning): Research Funding; TG Therapeutics: Consultancy, Research Funding; Juno/Celgene/BMS: Research Funding; BeiGene: Research Funding; ArQule/Merck: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; CATO/SMS Catapult: Research Funding; MEI Pharma: Research Funding; NeWave: Research Funding; AstraZeneca/Acerta: Research Funding; Pharmacyclics: Consultancy, Research Funding. Wu:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Qureshi:Janssen: Current Employment; Merck: Ended employment in the past 24 months. Upasani:Pharmacyclics: Current Employment, Current equity holder in publicly-traded company; Protagonist Therapeutics/Biomea Fusion: Current Employment, Current equity holder in publicly-traded company, Other: COI is for family member, Patents & Royalties. Naganuma:Pharmacyclics LLC, an AbbVie company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Mato:TG Therapeutics, Inc: Honoraria, Research Funding; Acerta: Research Funding; Dava: Honoraria; Pfizer: Research Funding; PerView: Honoraria; DTRM Biopharma: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; LOXO: Honoraria, Research Funding; Nurix: Research Funding; Pharmacyclics, LLC: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Medscape: Honoraria; BMS: Honoraria; BeiGene: Honoraria, Research Funding; Curio: Honoraria; Octopharma: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria; PER: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal