Abstract
Background: Although daratumumab-containing regimens improve the outcome of relapsed multiple myeloma (MM), the disease remains incurable. We recently published the outcome of daratumumab-based treatment in 583 relapsed myeloma patients in real-world practice (LeBlanc R et al. Br J Haematol 2022). Here we present outcomes of these patients at time of subsequent treatments for relapse immediately after their daratumumab-containing regimen.
Methods: We performed a retrospective study using the Canadian Myeloma Research Group Database (CMRG-DB), containing real-world data on outcomes for myeloma patients from Canadian academic centers. From this CMRG-DB, we previously identified 583 relapsed MM patients grouped in 4 daratumumab-containing regimens (DVd: n=143, DRd/p: n=263, DPd/p: n=77 and Dd/p: n=100). Expanding on the initial dataset, the objectives of this study were to benchmark clinical efficacy of subsequent treatment immediately following the daratumumab-containing regimens in terms of responses, progression-free survival (PFS) and overall survival (OS).
Results: Of the 583 relapsed MM patients receiving daratumumab-based therapy, after a median follow-up of 21 months (DVd: 16 months, DRd/p: 17 months, DPd/p: 28 months and Dd/p: 32 months), 178 patients are still on their daratumumab-based therapy, 116 patients died before subsequent therapy, 19 patients relapsed but did not initiated subsequent therapy and 2 patients declined subsequent therapy. Subsequent therapy was initiated in 268 patients following daratumumab-containing treatment. These patients were male in 55%, with a median age of 67.8 years (range 36.9-89.7 years) at time of post-daratumumab-containing therapy and had previously received 4 median lines of therapy (range 2-10) at that time. These patients were triple-class exposed in 97% and triple-class refractory in 59% including 89% refractory to lenalidomide; 66% of patients were previously exposed to autologous stem cell transplantation.
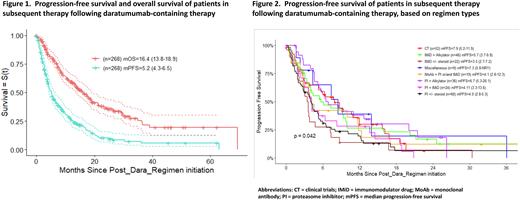
At the time of subsequent therapy, the median PFS and OS for the entire cohort were 5.2 months (95% CI 4.3-6.5) and 16.4 months (95% CI 13.8-18.9) respectively, as shown in Figure 1. Triple-class refractory patients (n=157) had a trend for a worst outcome with median PFS and OS of 4.7 months (95% CI 3.9-6.3) and 14.6 months (95% CI 11.5-18.9), respectively compared to 5.4 months (95% CI 4.1-8.0) and 17.8 months (95% CI 14.2-29.7) for non-triple-class refractory patients (n=111), with a p-value of 0.18 for PFS and 0.16 for OS.
Overall response rate for this entire cohort was 53% with 28% ≥VGPR. Based on regimens used at the time of subsequent therapy, higher ORR were achieved with immunomodulator drugs (IMiDs) and proteasome inhibitors (PIs) or IMiDs or PIs in combination with cyclophosphamide (Cy) compared to IMiDs or PI with steroids alone: ORR IMiD-Cy (n=46) 54%; PI-Cy (n=36) 66% and IMiD-PI (n=24) 63%. From patients treated with anti-CD38 monoclonal antibodies (daratumumab: n=18 and isatuximab: n=1) following daratumumab-containing regimens, ORR was 61% with 11% ≥VGPR; despite the fact that 90% of these patients were daratumumab-refractory. However, the median PFS for all treatment groups were shorter than 8 months, including those patients subsequently participating in a clinical trial as the next line of therapy [best subgroup achieving a median PFS of 7.9 months (95% CI 5.2-11.9)], as shown in Figure 2. Accordingly, the best OS was achieved in this same subgroup of patients participating in clinical trials (n=52) at time of relapse [median OS of 36.5 months (95% CI 21.1-not reached yet)].
Conclusion/summary: Our observations demonstrate that these poor results in real-world practice after daratumumab-based treatment gives rise to a high-risk patient population. We need effective novel therapies for such patients and dedicated prospective trials to better address their needs. Indeed, our results support participation to clinical trials where novel drugs/regimens are made available, as the best outcome is achieved in these patients relapsing following daratumumab-containing regimens. In the absence of this option, standard of care PIs and IMiDs remain the cornerstones of therapy but with limited long term disease control.
Disclosures
Leblanc:BMS: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; FORUS Therapeutics: Honoraria. Venner:Sanofi: Honoraria; BMS: Honoraria; GSK: Honoraria; Pfizer: Honoraria; FORUS Therapeutics: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Chu:Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. Jimenez-Zepeda:Takeda: Honoraria; GSK: Honoraria; Amgen: Honoraria; BMS: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; FORUS Therapeutics: Honoraria; Pfizer: Honoraria. McCurdy:Janssen: Honoraria; BMS: Honoraria, Research Funding; Sanofi: Honoraria; GSK: Honoraria; Forus: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Song:Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Celgene: Honoraria, Research Funding. Sebag:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Louzada:Pfizer: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Mian:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. White:Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Forus: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria. Stakiw:Janssen: Honoraria; FORUS Therapeutics: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Amgen: Honoraria. Kotb:Akcea: Honoraria; Amgen: Honoraria; BMS: Honoraria; Janssen: Honoraria; Karyopharm: Current equity holder in private company; Merck: Honoraria, Research Funding; Pfizer: Honoraria; Sanofi: Honoraria, Research Funding; Takeda: Honoraria; Celgene: Honoraria. Kaedbey:FORUS Therapeutics: Other: Advisory boards; Beigene: Other: Advisory boards; Pfizer: Other: Advisory boards; Jewish General Hospital, Montreal, QC, Canada: Current Employment; BMS. Janssen: Honoraria; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS. Janssen: Honoraria; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Other: Advisory boards; BMS: Honoraria, Other: Advisory boards; Janssen: Honoraria, Other: Advisory boards. Reece:Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Research Funding; BMS: Research Funding; Millenium: Research Funding; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy; Sanofi: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal