Abstract
Background: Varnimcabtagene autoleucel (IMN-003A) is an autologous CD19 directed CAR-T cell product with a 4-1BB co-stimulatory domain and a non-FMC63 murine single chain variable fragment (A3B1 binder), manufactured in India, which is being tested in the IMAGINE study (CTRI/2022/03/041162), a phase-2 clinical trial for patients (pts) with relapsed and/or refractory B cell malignancies (RR BCM). A fractionated infusion of 1 x 106 CAR+ cells (IMN-003A)/kg for B-ALL cohort and 5 x 106 CAR+ cells (IMN-003A)/kg for B-NHL cohort was administered over 3 days (D0, D+1, D+2) as 10%, 30% and 60% fractions after Fludarabine-Cyclophosphamide lymphodepletion regimen (LR). Safety and efficacy results are submitted in a separate abstract (#166297); here we present the early IMN-003A pharmacokinetic data and correlation with disease response.
Methods: IMN-003A was manufactured using cGMP compliant closed system (CliniMACS Prodigy®). The manufacturing data were obtained and analyzed, including % CAR transduction in the final product (FP) by flow cytometry (FC). Peripheral blood (PB) samples were obtained after consent at screening and as per patient schedule after infusion. Persistence of IMN-003A was evaluated in PB after infusion by ddPCR. Safety and efficacy data were collected for analysis. Bone marrow (BM) samples for MRD were obtained at screening, D+28 and D+90 for efficacy. Minimal residual disease (MRD) was performed by FC with 10-4 sensitivity. Apheresis, FP, and PB samples were analyzed for T-cell subpopulations and surface markers by FC.
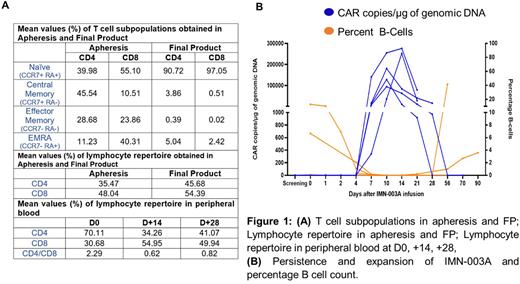
Results: Eight apheresis were obtained and transduced to achieve the final product with a median manufacturing time of 11d (range 10-18) with 100% success. The mean % transduction of IMN-003A on autologous T cells was 34.77% (range 15.49 - 58.35) with median transgene copies / genome of 2.25 (range 1.49 - 3.83). The mean CD4/CD8 ratio at apheresis and final product was 0.74 and 0.84 respectively. The T-cell subpopulations at apheresis and FP were analyzed (Figure 1A). The mean proportion of CD4 and CD8 positive naïve cells (CCR7+ RA+) was above 90% in the final product (Figure 1A). Median Product doubling time and Apheresis to Infusion time was 1.04d (range 1.02 - 1.38) and 20d (range 16 - 30) respectively. Post IMN-003A infusion, there was reversal of CD4/CD8 ratio in peripheral blood (Figure 1A).
IMN-003A CAR-T cells showed maximum PB expansion (Tmax) at median 10d (range 10 - 14) with median Cmax 180,680 CAR copies / µg gDNA (range 94,675 - 276,412). Among pts with 28-day follow-up, 80% had evidence of CAR+ T cells in PB, with median persistence not reached (range 28 - NR). B-cell aplasia was observed in all pts concurring with CART expansion (Figure 1B). Median loss of B cell aplasia was not reached (range 42 - NR), with no apparent association with response at D+28 and D+90. Hypogammaglobulinemia was noted in 6 of 7 evaluable pts and 2 pts received IVIg. Of evaluable patients with CD4+ T-cell counts below 200/μL, recovery (>200/μL) was seen at median of 10 days (range 2 - 10) after the IMN-003A cell infusion.
None of these pts have relapsed. Of 5 MRD-evaluable pts, response at day +28 (n=4) and +90 (n=1) was 100%. Updated results will be presented at the meeting.
Conclusion: In this industry-led first-in-India phase-2 study, IMN-003A production in a cGMP closed system was feasible and rapid. The peak of CAR-T expansion occurs at median 10d of infusion. The interim responses obtained are deep.
Disclosures
Jakka:Immuneel Therapeutics Private Limited: Current Employment. Dhar:Immuneel Therapeutics Private Limited: Current Employment. Joseph:Immuneel Therapeutics Private Limited: Current Employment. Kumar MG:Immuneel Therapeutics Private Limited: Current Employment. Palanisamy:Immuneel Therapeutics Private Limited: Current Employment. Kumar:Immuneel Therapeutics Private Limited: Current Employment. Soares:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Kamat:Immuneel Therapeutics Private Limited: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal