Abstract
Background: Prognostication of progression-free survival (PFS) and overall survival (OS) in adults with advanced stage classical Hodgkin lymphoma (A-HL) resulted in the International Prognostic Score (IPS7) (Hasenclever, 1998). The original IPS7 used dichotomous variables and was principally reflective of treatment from the 1980's. Analyses of more recently treated patients (pts) illustrated altered utility of the IPS7 in the modern era (Moccia, 2012), as well as a simplified score, the IPS3 (Diefenbach, 2015). We developed and validated a modern A-HL model, known as A-HIPI, to predict PFS & OS by 5 years (y) using comprehensive individual patient data, including continuous data values, from international clinical trials and large prospective HL registries that were standardized, normalized & harmonized as part of the HoLISTIC Consortium.
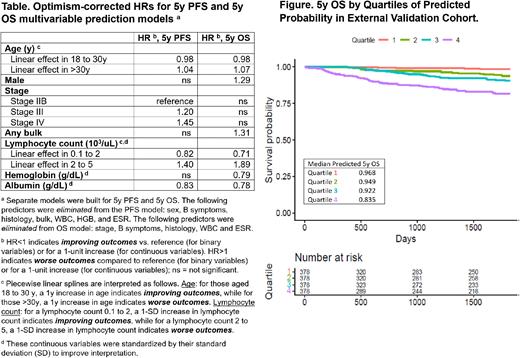
Methods: Model development was performed on a cohort of 4,027 pts treated on 8 international A-HL clinical trials, from 1996-2012 (ECOG 2496, SWOG 0816, HD2000, HD9601, HD0607, HD0801, UK Stanford V, RATHL). External validation was performed in a separate cohort of 1,512 A-HL pts treated with curative intent from 4 HL registries (BC Cancer, Princess Margaret Cancer Centre, Iowa/Mayo SPORE, Australia). Untreated A-HL pts with stage IIB, III or IV disease & ages 18-65y were included. The primary outcomes of 5y PFS & 5y OS were estimated with separate Cox models. Baseline predictors were: sex, stage, B symptoms, histology, any bulk (by trial definition), and continuous forms of age, white blood cell count, lymphocyte count (LC), hemoglobin, albumin & erythrocyte sedimentation rate. Multiple imputation was used for missing data. To consider possible non-linear relationships for continuous variables (age & all lab values), we examined plots & splines. We used backwards elimination (P<0.05) to develop the model. Internal validation with 200 bootstrap samples was conducted to estimate optimism and correct for overfitting. The final prediction equations applied optimism corrections to beta coefficients & hazard ratios (HR). The model was evaluated in the external validation cohort & compared using C-statistics. Model scores were also grouped by quartiles for visualization in Kaplan Meier (KM) curves.
Results: Median age in the development cohort was 33y; 55% were male; nodular sclerosis was the most common histology (74%); 35% had any bulk; 73% B symptoms; and stage was distributed as 28% IIB, 39% III & 33% IV. KM estimators were 0.77 (861 events by 5y) for 5y PFS and 0.92 for 5y OS (280 events by 5y). Median follow-up was 52 months (Q1=36, Q3=90). See the Table for predictors included in the prediction models & optimism-corrected HRs. Of note, continuous age & LC values were both modeled with piecewise linear splines, which yielded non-linear, U-shaped relationships with PFS & OS (Table). Optimism-corrected C-statistics in the development model were 0.60 for 5y PFS & 0.71 for 5y OS. Most baseline characteristics and outcomes of the external cohort were similar besides a difference in stage (38% IIb, 30% III, 32% IV) and longer median follow-up (74 months, Q1=27, Q3=133) in the validation cohort. C-statistics for the A-HIPI model were 0.58 for 5y PFS and 0.72 for 5y OS in external validation. In comparison, C-statistics were 0.58 & 0.57 for 5y PFS and 0.66 & 0.66 for 5y OS for the IPS7 and IPS3, respectively. KM visualization of the grouped A-HIPI model quartile scores for OS is shown (Figure).
Conclusion: Via state-of-the-art predictive modeling, we developed & externally validated the A-HIPI among >5,500 newly diagnosed A-HL pts from recent seminal clinical trials & prospective HL registries. We found unique prognostic variables significant for PFS vs. OS. Furthermore, we identified novel non-linear, U-shaped relationships between age and LC with survival. Collectively, the model will estimate 5y PFS & OS for A-HL pts based on individualized baseline values. A web-based calculator will be presented at ASH to simplify application of the A-HIPI. Despite inclusion of continuous variables (and non-linear relationships), there was modest enhancement in model performance for PFS vs. the IPS. However, we demonstrated meaningful improvement in prediction of 5y OS. Future modeling will include incorporation of differing treatment regimens & response-adapted imaging via multistate modeling to improve prediction of survival and also delineation of treatment-related late effects.
Disclosures
Hawkes:Regeneron: Speakers Bureau; Janssen: Speakers Bureau; Astra Zeneca: Speakers Bureau; Merck Sharpe and Dohme: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Bristol_Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Merck KgA: Research Funding; Bristol-Myers Squibb: Research Funding; Roche: Research Funding; Roche: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Link: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics: Consultancy. Johnson:BMS: Honoraria; Boehringer Ingelheim: Consultancy; Oncimmune: Consultancy; Novartis: Honoraria; Genmab: Honoraria; Incyte: Honoraria; Kite Pharma: Honoraria; Kymera: Honoraria; MorphoSys: Honoraria; Celgene: Honoraria; Janssen: Consultancy; Epizyme: Consultancy, Research Funding; Takeda: Honoraria. Link:MEI: Consultancy; Novartis: Research Funding; Jannsen: Research Funding; Bristol-Myers Squibb: Research Funding; Genentech / Roche: Consultancy, Research Funding. Savage:BMS, Janssen, Kyowa, Merck, Novartis, and Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene and Regeneron: Membership on an entity's Board of Directors or advisory committees. Zinzani:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Maurer:Morphosys: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Research Funding; BMS: Research Funding. Evens:MorphoSys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal