Abstract
Background: Hematopoietic stem cell transplant (HCT) represents the most effective mean to achieve cure for children, adolescents and young adults with relapsed or refractory (R/R) B-ALL. Among the several disease-specific and transplant-specific characteristics evaluated so far, achievement of minimal residual disease (MRD) negative complete remission (CR) prior to HCT is the strongest independent prognostic factor of post-HCT outcome. Antigen directed therapies (ADT) such as blinatumomab, inotuzumab ozogamicin and chimeric antigen receptor (CAR) T-cell are very effective at inducing MRD-negative remission and offer an alternative approach for disease control in patients with chemo-resistant disease. We hypothesized that ADT may provide a more durable disease control and lesser morbidity than conventional chemotherapy (CT) and therefore we sought to explore whether the type of treatment (ADT or CT) adopted to achieve MRD negative CR prior to HCT, impacts post-HCT outcomes.
Patients and methods: This is a retrospective, multi-institutional cohort study of patients (pts) with R/R B-ALL who received an allogeneic HCT in MRD negative CR between 2010-2021. Patients were eligible if they were ≤30 years at time of diagnosis of R/R B-ALL, had received either CT or ADT (blinatumomab, CAR T-cell, inotuzumab ozogamicin with or without preceding chemotherapy) for the treatment of R/R B-ALL and had achieved MRD negative CR (<0.01% blasts by flow cytometry) prior to HCT. Patients with isolated extramedullary relapse or acute leukemia of ambiguous lineage or history of prior HCT were excluded. Patients were categorized as being in the "CT" or "ADT" group based on whether they received conventional CT or ADT as last cycle that led to MRD negativity prior to HCT. The primary objective of the study was to compare the EFS, OS and treatment related mortality (TRM) from time of transplant according to the therapy (ADT vs. CT) received pre-HCT. Kaplan-Meier or Aalen-Johansen estimates were used to estimate survival rates and curves were compared using logrank or Gray's tests.
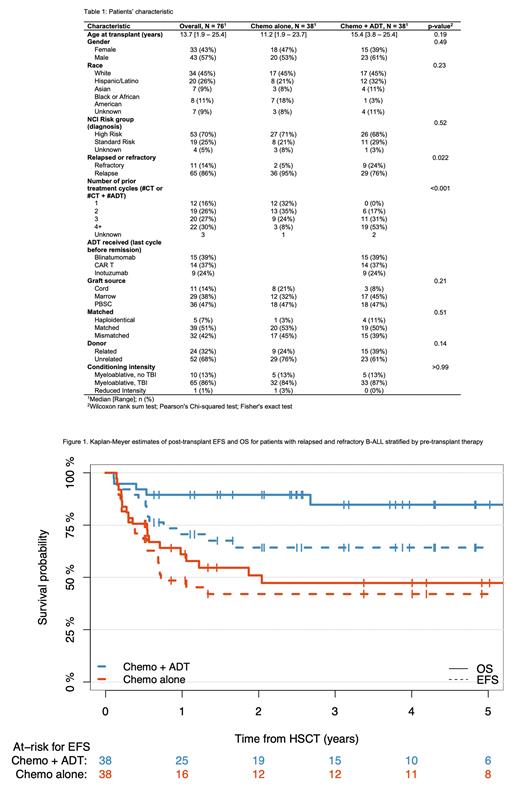
Results: Seventy-six pts from 7 centers met eligibility criteria; 38 pts (50.0%) received ADT and 38 pts (50.0%) received chemotherapy as last cycle prior to HCT. Demographic, disease and treatment characteristics are summarized in Table 1; notably, more pts in the ADT group had refractory leukemia and more pts had received ≥4 cycles of therapy before achieving MRD negative remission in the ADT group than in the CT group. The 5-yr. EFS and OS for the entire cohort were 53% and 67%, respectively. EFS was superior in the ADT group compared to the CT group (5-yr. EFS 64% vs. 42%, p=0.04; Figure 1). OS was also superior in the ADT group (85% vs. 47%, p=0.0008; Figure 1). In a univariable and multivariable prognostic model for OS that included age, race, having relapsed or refractory disease and CT or ADT as pre-HCT therapy, pre-HCT therapy was the only significant prognostic factor (p<0.001); in a similar model for EFS, pre-HCT therapy was the only significant prognostic factor in the univariable analysis (p=0.04) but not in the multivariable analysis. There was no difference in the incidence of acute GVHD, rate of immune reconstitution at day 100, time to engraftment and cumulative incidence of relapse between the two groups. However, the CT group appeared to have a higher rate of both TRM (33.0% vs. 8%, p=0.01) and relapse related mortality, although not significant for the latter (19% vs. 7% p=0.12).
Conclusion: This multicenter study shows that having received ADT prior to HCT may be beneficial when compared to conventional chemotherapy for patients with R/R B-ALL who receive a HCT in MRD negative CR. Patients who received ADT experienced a superior EFS and OS primarily due to a significantly lower TRM, despite having received more cycles of therapy prior to proceeding to HCT. There was no difference in the relapse rate among the two groups further supporting the notion that MRD negative CR is the single most important predictor of post-HCT relapse. Further analysis is ongoing to identify specific factors that could explain the difference in post-HCT mortality among the 2 groups.
Disclosures
Raetz:Pfizer: Research Funding; BMS: Other: Data and Safety Monitoring Board. Hijiya:Novartis: Research Funding; Pfizer: Research Funding; Incyte: Honoraria, Other: Data monitoring committee; Stemline Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Satwani:Takeda: Honoraria; Mesoblast: Honoraria. Curran:Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal