Abstract
INTRODUCTION: International Myeloma Working Group (IMWG) criteria require 24-hour urine specimens as part of response assessments, which can be cumbersome for patients to collect. This requirement is particularly onerous before autologous stem cell transplantation (ASCT), a period relatively soon after diagnosis with many concurrent testing requirements. The necessity of urine testing to complete response (CR) determinations and its prognostic impact on progression-free survival (PFS) have been questioned in subgroup analyses of clinical trials (Dejoie 2016; Lahuerta 2019). However, the real-world importance of urine testing to IMWG response criteria more broadly has not been ascertained.
METHODS: We analyzed all patients with multiple myeloma (MM), excluding those with oligosecretory disease or progressive disease during induction, who underwent first ASCT at our institution between 2016-2019. Patients with measurable serum M-protein (>1 g/dL) and light-chain (LC)-only disease were included. Patients with missing diagnostic or pre-ASCT data precluding IMWG response assessments were excluded. Evaluable urine studies were defined as a documented urine immunofixation (UIFE) value or, if UIFE was missing, a presumptive +UIFE if urine protein electrophoresis showed a paraprotein. For patients with a documented negative UIFE at diagnosis, -UIFE was presumed if urine studies were not repeated before ASCT. For patients with evaluable baseline and pre-ASCT urine testing, we assessed responses using both traditional IMWG criteria and 'urine-free’ IMWG criteria, whereby urine testing results were systematically removed as options or requirements to achieve any given response depth. We hypothesized that urine-free criteria would yield discordant responses in <10% of patients, a proportion we determined a priori to be clinically irrelevant.
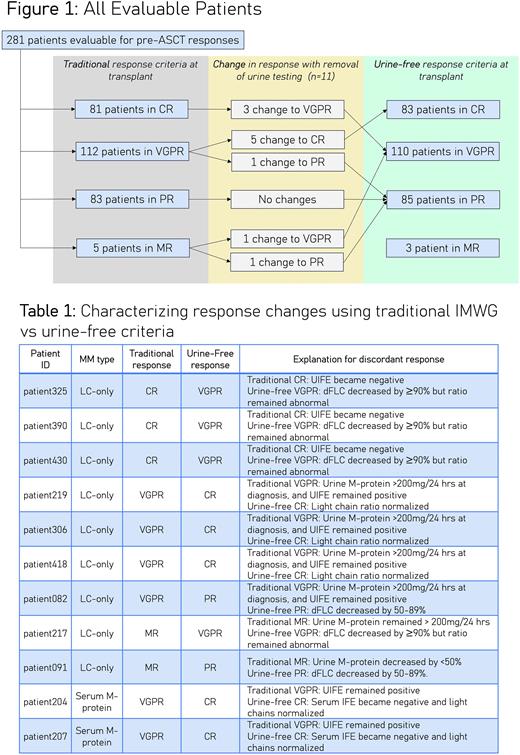
RESULTS: Of 347 screened patients, 66 (19%) were excluded due to missing data precluding pre-ASCT response assessments by traditional IMWG criteria. Compared to patients (n=281) with sufficient data, these 66 excluded patients were more likely to have LC-only disease and to have been transplanted before 2018. Of 281 patients evaluable for responses by both traditional and urine-free IMWG criteria, 11 patients (4%) had discordant responses (Figure 1). Discordance rates were 14% (9/63) among patients with LC-only disease and 1% (2/218) among patients with measurable serum M-proteins. Of these 11 discordant responses, 7 were superior with urine-free criteria while 4 were inferior (Table 1).
DISCUSSION: In our retrospective study, response depths changed in only 4% of patients when urine requirements were systematically removed from IMWG criteria. Rates of discordance varied by paraprotein biology: 1% of patients with a measurable serum M-protein had discordant responses, versus 14% of patients with LC-only disease. This calls into question the common practice of requiring repeat 24-hour urine assessments for all patients prior to ASCT, since its utility may be practically limited to patients with LC-only disease. Interestingly, omitting urine from IMWG response criteria did not uniformly shift responses in either direction. Limitations of our data include missing data in 66 patients (who had predominantly LC-only disease) as well as lack of generalizability to ASCT-ineligible patients. However, our study is important in demonstrating that 24-hour pre-ASCT urine testing may be unnecessary for many patients with MM, particularly those with a measurable serum M-protein at diagnosis. Further research is needed to assess whether urine-free or traditional response criteria better reflect underlying disease biology in terms of their ability to prognosticate PFS.
Disclosures
Knoche:Amgen: Honoraria. Chung:Janssen: Research Funding; Cellectis: Research Funding; BMS/Celgene: Research Funding; AbbVie: Research Funding; Merck: Research Funding; Caelum: Research Funding; Sanofi: Honoraria. Martin:Legend Biotech: Consultancy; Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding; GlaxoSmithKline and Legend Biotech: Consultancy. Wolf:Adaptive Biotechnologies: Consultancy. Wong:Janssen: Research Funding; GSK: Research Funding; Fortis: Research Funding; Genentech: Research Funding; Caelum: Research Funding; Bristol Myers Squibb: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Patient Discovery: Research Funding; Catalent Biologics: Consultancy; Dren Bioscience: Consultancy. Shah:CSL Behring: Consultancy; Allogene: Consultancy; AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; TeneoBio: Research Funding; Nektar: Research Funding; Amgen: Consultancy; BMS/Celgene: Consultancy, Research Funding; CareDx: Consultancy; GSK: Consultancy; Oncopeptides: Consultancy; Indapta Therapeutics: Consultancy; Kite: Consultancy; Janssen: Research Funding; Karyopharm: Consultancy; Sutro Biopharma: Research Funding; Poseida: Research Funding; Sanofi: Consultancy; Bluebird Bio: Research Funding; Precision Biosciences: Research Funding. Banerjee:Curio Science: Honoraria; Eradigm Consulting: Consultancy; Guidepoint Global: Consultancy; Sanofi: Consultancy; SparkCures: Consultancy; Pack Health: Research Funding; i3 Health: Consultancy; Clinical Care Options: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal