Abstract
Introduction: Mantle cell lymphoma (MCL) is a rare, aggressive form of non-Hodgkin's lymphoma with relatively poor prognosis compared to other B-cell malignancies, high economic burden, and a changing treatment landscape with recent therapeutic advances. This study describes current treatment patterns, healthcare resource use (HRU), and costs in patients with MCL in the US.
Methods: Adult patients with ≥2 diagnosis codes for MCL on ≥2 distinct dates within 12 months were identified in Optum Clinformatics claims data from 10/1/2015 to 3/31/2021. The first observed MCL diagnosis was considered the index date. The baseline period was defined as the 12 months of continuous enrollment pre-index; continuous enrollment was also required for ≥1 month post-index. Patients with diagnosis codes for MCL or other malignancies during baseline were excluded. The study period spanned from the index date till the earliest of either end of continuous enrollment or end of data availability. Demographic and clinical characteristics were described during the baseline period. All-cause and MCL-related HRU in terms of monthly incidence rates and 95% confidence intervals (CI) and per patient per month (PPPM) costs were described during the study period. A subset of patients meeting the following additional criteria were included in the treatment sequence analysis: no claims for treatments of interest during the baseline period and ≥1 claim for treatments during the study period. Treatments of interest included National Comprehensive Cancer Network (NCCN)-recommended systemic therapies, stem cell transplantation (SCT), and chimeric antigen receptor (CAR)-T therapy. An algorithm was developed to identify treatments in first line (1L), second line (2L), and third line (3L) during the study period. HRU and costs were described during the 1L, 2L, and third and subsequent line (3L+) periods.
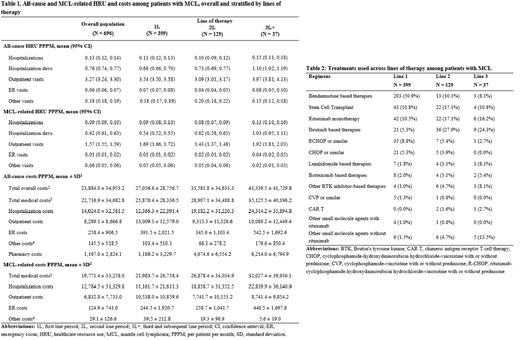
Results: The study included 696 patients with MCL, with a mean follow-up time of 22 months. Patients had a mean age of 71 years and were predominantly male (68%), with most having Medicare coverage (76%). The most common comorbidities during baseline were cardiovascular disease (29%), diabetes (27%), and chronic obstructive pulmonary disease (24%), with a mean National Cancer Institute Comorbidity Index (NCICI) of 1.9. During the study period, there were 0.13 (95% confidence interval: 0.12, 0.14) all-cause hospitalizations, 3.27 outpatient visits (3.24, 3.30), 0.06 (0.06, 0.07) emergency room (ER) visits, and 0.18 (0.18, 0.19) other visits PPPM (Table 1). Majority of hospitalizations were MCL-related (0.09 PPPM). MCL-related outpatient visits (1.57 PPPM), ER visits (0.01 PPPM), and other visits (0.06 PPPM) formed a lower proportion of all-cause visits. Patients had considerable total all-cause costs ($23,884 PPPM), almost all of which were medical costs ($22,717 PPPM). MCL-related medical costs contributed substantially to all-cause costs ($19,771 PPPM), largely due to MCL-related hospitalizations ($12,785 PPPM). 399 patients (57%) who received a treatment of interest during the study period were included in the treatment pattern analysis. Among these patients, 129 (32%) were observed to receive a treatment of interest in 2 or more lines. Bendamustine-based therapies were most used in 1L (51%), followed by SCT and rituximab monotherapy (11% each) (Table 2). Ibrutinib-based therapies were the most common in 2L (28%) and 3L (24%). Mean treatment duration decreased with increasing lines of therapy (173 days for 1L, 154 days for 2L, and 139 days for 3L). All-cause and MCL-related HRU and costs tended to increase with increasing lines of therapy (Table 1) - for example, hospitalization costs were twice as high in 3L+ ($24,314 PPPM for all-cause and $22,840 PPPM for MCL-related hospitalizations) than in 1L ($12,366 PPPM for all-cause and $11,162 PPPM for MCL-related hospitalizations).
Conclusions: This study describes the contemporary treatment patterns and economic burden for MCL in the US. Results show that a range of treatments are used to manage MCL, with more recently approved therapies such as ibrutinib being used more commonly in later lines. This study also highlights high HRU and cost burden among patients with MCL, which increases with subsequent lines of therapy.
Disclosures
Garg:Merck & Co., Inc.: Current Employment. Satija:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Song:Gamida Cell, Inc.: Consultancy; Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Sarpong:Merck & Co., Inc.: Current Employment. Meade:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Lemus-Wirtz:Merck & Co., Inc.: Consultancy, Other: At the time of this research, I was an employee of Analysis Group, Inc., which received consulting fees from Merck. Gaburo:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Signorovitch:Gamida Cell, Inc.: Consultancy; Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Raut:Merck & Co., Inc.: Current Employment. Ryland:Merck & Co., Inc.: Current Employment. Chakraborty:Merck & Co., Inc.: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal