Abstract
Introduction: A number of variables have been shown to predict survival outcomes in follicular lymphoma (FL), including the Follicular Lymphoma International Prognostic Index (FLIPI) score measured at initial diagnosis and progression of disease within 24 months (POD24). However, there exists little research aimed at informing prognosis in a cohort comprised exclusively of FL patients whose disease has relapsed after prior therapy. To address this gap, we assessed the FLIPI score measured at diagnosis and POD24 with respect to their ability to stratify survival outcomes measured from the time of first progression in a group of individuals with FL who experienced disease progression after initial treatment.
Methods: An external validation of the FLIPI score measured at diagnosis and POD24 was conducted using population-level data from Alberta, Canada. The study cohort was comprised of individuals diagnosed with FL between 2004-2010 who received either systemic therapy and/or radiation within 180 days of diagnosis and who subsequently had disease progression. Individuals were followed until death or last-known contact with the healthcare system. Relevant data were identified through record linkage of the provincial cancer registry and electronic medical records along with a manual chart review conducted by trained medical doctors. Outcomes were examined from the time of first progression and included time to death (OS), time to death after first progression or second progression (PFS2), and time to death or initiation of third line treatment (TTNT2). For each outcome, Kaplan-Meier curves were estimated stratified by FLIPI and by POD24. To quantify the degree of separation of the Kaplan-Meier curves, we estimated hazard ratios (HRs) using a Cox proportional hazards model and Harrell's concordance statistic (c-statistic) for both the FLIPI index and POD24.
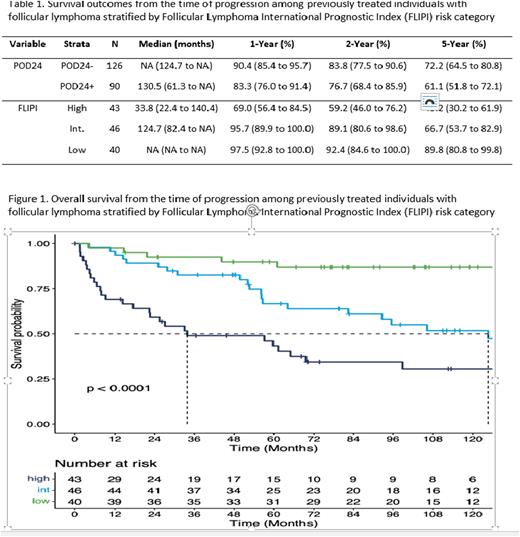
Results: 910 individuals were diagnosed with FL between 2004-2010, of which 584 received systemic or radiation therapy. 527 individuals initiated front-line therapy within 180 days of diagnosis, of which 216 experienced progression during the follow-up period and were included in these analyses. Among the 216 individuals, 162 (75%) received front-line systemic therapy which was primarily R-CVP (n=57; 35%) or R-CHOP (n=78; 48.1%). From the time of disease progression, median OS was 11.7 years (95% CI: 8.9-NA). POD24 status was available for all 216 individuals, with 90 (42%) individuals having evidence of progression of disease within 24 months of initiating front-line therapy (POD24+). Overall, 129 of the 216 (60%) individuals had complete information required to derive the FLIPI risk score with 43 (33%) being classified as high-risk, 46 (36%) intermediate-risk, and 40 (31%) low-risk. OS estimates stratified by FLIPI score and by POD24 are presented in Table 1. POD24 was not highly predictive of OS from the time of disease progression (c-statistic: 0.55; HR [POD24+ vs. POD24-]: 1.42; 95% CI: 0.93 to 2.15; p = 0.10). Conversely, the FLIPI score measured at diagnosis was highly predictive of OS from the time of disease progression (c-statistic 0.70; HR [High vs. Low]: 7.38; 95% CI: 3.05 to 17.88, p < 0.01; HR [Int. vs. Low]: 3.70; 95% CI: 1.51 to 9.10, p < 0.01; ; HR [High vs. Int.]: 1.99; 95% CI: 1.15 to 3.47, p = 0.01; Figure 1). These findings were similar when examining PFS2 and TTNT2. In sensitivity analysis, findings were similar when restricting to individuals who received front-line systemic therapy. In addition, the classification of individuals by both POD24 and FLIPI did not meaningfully improve discrimination over FLIPI alone.
Conclusions: The FLIPI score measured at initial diagnosis can be used for risk stratification at the time of disease progression among previously treated FL patients. In contrast, POD24 was not highly predictive of survival outcomes from the time of disease progression within this population of FL patients who all had disease progression. Future research is needed to determine if the prognostic utility of the FLIPI risk score measured at initial diagnosis can be improved upon by incorporating variables assessed at the time of relapse.
Disclosures
Owen:Incyte: Honoraria; GIlead: Honoraria; Roche: Honoraria; Merck: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; BeiGene: Honoraria. Chua:Gilead: Honoraria; Abbvie: Honoraria; Seagen: Honoraria; Merck: Honoraria; Amgen: Honoraria; Incyte: Honoraria; Bayer: Honoraria; Pfizer: Honoraria. Boyne:Janssen Inc.: Research Funding. Joe-Uzuegbu:Janssen Inc.: Research Funding. Shakir:Janssen Inc.: Research Funding. Gonga:Janssen Inc: Research Funding. Brenner:Janssen Inc.: Research Funding. Elia-Pacitti:Janssen Inc.: Current Employment, Current equity holder in publicly-traded company. Ewara:Janssen Inc.: Current Employment, Current equity holder in publicly-traded company. Cheung:Janssen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal