Abstract
Introduction More than 130 drugs have been suspected to induce autoimmune hemolytic anemia (AIHA), listed in 2014 (Garratty et al. Immunohematology). Several mechanisms have been implicated, from drug-dependent autoantibodies to non-specific stimulation of the immune system. Some cases of drug-induced AIHA (DI-AIHA) have been experimentally demonstrated. However, most drugs are suspected from case-reports with the exclusion of other causes of secondary AIHA, time from drug initiation to AIHA onset compatible with an immune mechanism and AIHA recovery after drug withdrawal. However, comparative studies assessing the risk of DI-AIHA in the population are lacking. The aims of this two-step study were 1) to detect new signals of drugs that may responsible for DI-AIHA in Vigibase®, the World PharmacoVigilance which record more than 20 x 106 cases of adverse drug reaction (ADRs), and 2) to assess the association between the drugs listed in 2014 as well as of new potential drugs responsible for DI-AIHA identified in Vigibase® and the occurrence of AIHA in a nationwide cohort study in France.
Methods The first step of the study was a case/non-case study among ADRs recorded up to February 2020 in Vigibase®. The frequency of exposure to each drug was compared between cases of AIHA and other reported ADRs, resulting in the calculation of Reporting Odds Ratios (RORs) and their 95% confidence interval (95% CI). Drugs with a ROR >1 were defined as signals of drugs possibly inducing DI-AIHA.
The second step of the study was conducted in the AHEAD cohort, consisting in all patients with incident AIHA in France selected between 2012-2018 in the French national health database. This database links sociodemographic, out-of-hospital and hospital data for the entire French population (67 million individuals). The date of AIHA diagnosis was defined by the first hospital discharge diagnosis or long-term disease (recorded by general practitioners) of AIHA occurring after a prior observation period of at least two years. Patients were identified using the D59.0 and D59.1 codes of the international classification of diseases, version 10. Patients with secondary AIHA were excluded. We conducted a case-crossover (CCO) analysis. This is a self-controlled design where each case serves as its own control to overcome non time-dependent confounding factors. The exposures to drugs form the 2014 list and new drugs identified at step 1 were searched in out-of-hospital dispensing data. The exposure to each drug during the six weeks before AIHA (case period) was compared to the exposure during a six-week control period three months before. Patients hospitalized during case or control periods were excluded from analyses. To overcome exposure trend and protopathic bias, we also performed a bidirectional CCO analysis by including an immediate post-event control period of 6 weeks.
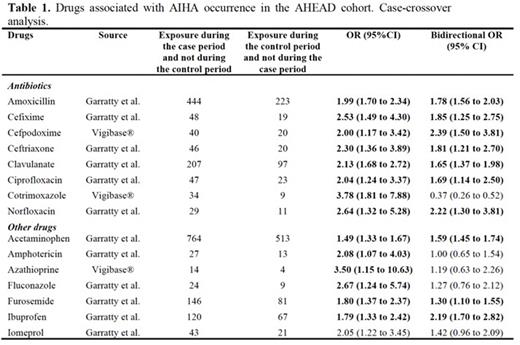
Results During the study period, 3,371 cases of DI-AIHA were recorded in Vigibase®. Eighty new signals were identified. The most frequent drug classes were antibiotics, corticosteroids, non-steroidal anti-inflammatory drugs, vaccines, immunosuppressive agents, anticancer and cardiovascular drugs. Overall, the association between 160 suspected drugs marketed in France during the study period and AIHA occurrence was assessed using the CCO design in the AHEAD cohort (n=4,722 patients with incident primary AIHA). We identified an association between AIHA occurrence and some antibiotics, ibuprofen, acetaminophen and furosemide (Table 1).
Conclusion This study identified new signals of DI-AIHA using worldwide pharmacovigilance data, and confirmed the risk at the population level for some antibiotics, ibuprofen, acetaminophen and furosemide.
Disclosures
Michel:argenx: Other: Boards, speaker at educational sessions; Sobi: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions; UCB: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Moulis:Grifols: Other: Boards, speaker at educational sessions, Research Funding; Novartis: Other: Boards, speaker at educational sessions, Research Funding; Argenx: Other: Board; Amgen: Other: Boards, speaker at educational sessions; Sobi: Other: Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal