Abstract

Introduction: Long-term, untreated anemia in patients with non-transfusion-dependent (NTD) β-thalassemia can lead to serious clinical complications. Several studies have shown that increasing hemoglobin (Hb) levels by ≥ 1 g/dL can significantly decrease the risk of developing morbidities (Musallam KM, et al. Ann Hematol 2022;101:203-204; Musallam KM, et al. Ann Hematol 2021;100:1903-1905). Although there are no available treatments for NTD β-thalassemia-associated anemia, previous studies showed that luspatercept improved erythropoiesis leading to a decrease in transfusion burden in pts with transfusion-dependent β-thalassemia, and durably increased Hb levels in pts with NTD β-thalassemia (Cappellini MD, et al. N Engl J Med 2020;382:1219-1231; Taher AT, et al. HemaSphere 2021;5[Suppl 2]. Abstract S101).
Here, we report the long-term efficacy update of luspatercept in patients with NTD β-thalassemia in the BEYOND trial (NCT03342404).
Methods: Eligible patients (N = 145) with NTD β-thalassemia or HbE/β-thalassemia (defined as receiving 0-5 RBC units in the 24 weeks [wk] prior to randomization) and Hb levels ≤ 10 g/dL were randomized to luspatercept (1.0-1.25 mg/kg) or placebo subcutaneously for ≥ 48 wk. Assessment groups were based on patient assignment at randomization: patients in the luspatercept arm were assessed while remaining on treatment and patients in the placebo arm were assessed up to discontinuation or crossover to luspatercept. Mean change from baseline in Hb was assessed for continuous 12-wk intervals up to wk 144. Erythroid response was defined as mean Hb change from baseline of ≥ 1 g/dL and was assessed over rolling 12-wk intervals. The incidence of RBC transfusions (events and units) was also assessed.
Results: As of September 22, 2021, 83 (86.5%) patients in the luspatercept arm and 16 (32.7%) in the placebo arm completed ≥ 96 wk of treatment. Moreover, 72 (75.0%) patients in the luspatercept arm completed ≥ 120 wk of treatment. The median (range) treatment duration was 150.1 (15.0-185.4) wk in the luspatercept arm and 61.1 (3.0-138.0) wk in the placebo arm. Patients in the luspatercept and placebo arms received a median (range) of 42.0 (3.0-61.0) doses and 20.0 (1.0-46.0) doses per patient, respectively.
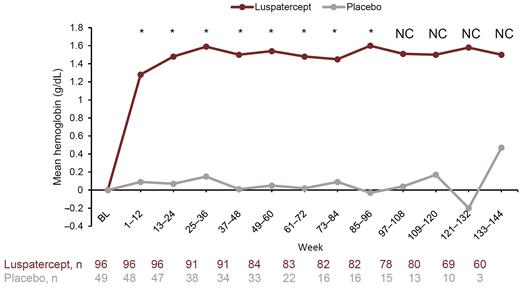
Mean (standard error [SE]) Hb change from baseline in the luspatercept arm was 1.28 (0.069) g/dL during wk 1-12 and 1.48 (0.078) g/dL during wk 13-24, and the increase was maintained among patients remaining on study through all time points. Improvements in Hb levels from baseline were nominally significant versus placebo at assessed 12-wk intervals up to wk 96 (Figure).
The proportion of patients experiencing an erythroid response during any 12-wk interval increased from 91.7% (88/96 patients) at the primary data cutoff date (September 14, 2020), to 93.8% (90/96 patients) at the current data cutoff date. Among responders, the proportion of patients in the luspatercept arm with more than one 12-wk rolling response increased from 35.2% (31/88 patients) to 61.1% (55/90 patients) between the primary and current data cutoff dates, and the mean (standard deviation [SD]) total duration of erythroid response among patients with at least one 12-wk rolling response also increased, from 611.1 (243.3) to 873.1 (363.4) days.
During wk 1-96, a smaller proportion of patients in the luspatercept arm compared to the placebo arm (10.4% [10/96 patients] vs 32.7% [16/49 patients]) had ≥ 2 RBC transfusions. During the same period, the mean number of transfusion events (0.4 vs 1.3) and RBC units transfused (0.7 vs 2.2) per patient was lower in the luspatercept arm than in the placebo arm. During wk 97-144, the mean number of transfusion events and units per patient in the luspatercept arm remained stable (0.2 events and 0.2 units, respectively).
Conclusions: Long-term luspatercept treatment resulted in sustained and significantly improved Hb levels in patients with NTD β-thalassemia. The duration of erythroid response increased with an additional year of luspatercept treatment. Few patients required RBC transfusions and the cumulative incidence of RBC transfusions remained low and relatively stable through 144 wk.
Disclosures
Taher:Vifor Pharma: Consultancy, Research Funding; Ionis Pharmaceuticals: Consultancy, Research Funding; Imara: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Cappellini:BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Vertex: Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Silence: Membership on an entity's Board of Directors or advisory committees. Piga:Celgene: Honoraria; Chiesi: Honoraria. Porter:Celgene: Consultancy, Honoraria; Agios: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; La Jolla Pharmaceuticals: Honoraria; Protagonism: Honoraria; VIFOR: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Coates:Forma: Consultancy; Chiesi: Consultancy; Bluebird: Consultancy; Vifor: Consultancy; BMS Pharma: Consultancy; Agios: Consultancy. Musallam:Vifor Pharma: Consultancy; Agios Pharmaceuticals: Consultancy; Celgene Corp (Bristol Myers Squibb): Consultancy; Novartis: Consultancy; CRISPR Therapeutics: Consultancy; Pharmacosmos: Consultancy. Forni:Novartis: Consultancy, Research Funding, Speakers Bureau; BMS (Celgene): Consultancy, Research Funding, Speakers Bureau; Vertex: Consultancy, Speakers Bureau. Shetty:Celgene/Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bosilkovska Weisskopf:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company; Vifor Pharma: Other: Husband's current employer. Wei:BMS: Current Employment. Kuo:Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kattamis:BMS/Celgene: Consultancy, Honoraria, Research Funding; Chiesi: Honoraria; VERTEX: Consultancy, Honoraria; IONIS: Consultancy; AGIOS Pharmaceuticals: Consultancy; ViFOR: Consultancy; AMGEN: Consultancy; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal