Abstract
Background Crizanlizumab and voxelotor are new disease modifying therapies that have been shown respectively as single agents to reduce the frequency of vaso-occlusive crises (VOCs) and improve anemia. Clinical effects of these drugs in combination have not been examined in clinical trials.
Objectives Evaluate the rates of VOC-related acute care utilization, lengths of stay (LOS) of hospitalizations and hemoglobin levels in sickle cell disease (SCD) patients treated with crizanlizumab, voxelotor, or both.
Methods A single center, retrospective chart review was performed of all adult SCD patients to identify those treated with crizanlizumab and voxelotor, alone and in combination, for at least 12 months. Number of acute care utilization visits which includes emergency department (ED) visits, infusion room visits and hospitalizations for management of VOCs, lengths of stay of hospitalizations, as well as steady state hemoglobin values in the 12 months before and after initiation of crizanlizumab, voxelotor, or both were obtained for each patient. Acute care visits and hospitalizations for management of conditions other than acute VOC were excluded. A descriptive analysis was conducted to summarize patient characteristics and outcomes including changes from baseline over time for the three treatment groups separately. Fisher's exact tests were applied to compare categorical variables among the three treatment groups. Kruskal-Wallis tests were used instead for outcomes at a given time point or changes from baseline. A significant Kruskal-Wallis test was followed by pairwise Dunn tests adjusted for multiple testing using the Benjamini-Hochberg method. P-values smaller than 5% were considered statistically significant. All the statistical analyses were performed in R version 4.1.2.
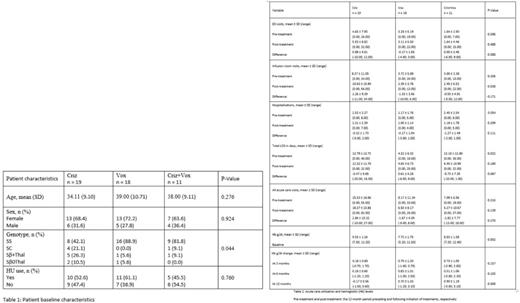
Results Of 266 adult SCD patients, 77 (29%) had ever taken crizanlizumab, voxelotor or both. 48 out of 77 (62%) patients met inclusion criteria of being on these therapies alone or in combination for at least 12 months. Of the included patients, 19 patients were treated with crizanlizumab alone, 18 patients were treated with voxelotor alone, and 11 patients were treated with both crizanlizumab and voxelotor. Baseline characteristics are summarized by treatment group in Table 1. Patients on crizanlizumab alone had a broader distribution of hemoglobin genotypes with a significantly smaller proportion having the Hb SS genotype (42.1%) compared to those on voxelotor alone (88.9%) or on a combination of crianlizumab and voxelotor (81.8%). Age, gender, and hydroxyurea (HU) use were not statistically significantly different among the three treatment groups.
There was no statistically significant difference in acute care utilization in patients on crizanlizumab, voxelotor, or both (Table 2). Specifically, there was no evidence of difference in VOC related ED visits, infusion room visits, hospitalizations and LOS in the 12 months following initiation of these therapies compared to the 12 months preceding it. Of note, in patients on both crizanlizumab and voxelotor, the mean number of VOC related hospitalizations before initiation of treatment was 2.45 ± 2.54 compared to 1.18 ± 1.78 after, although these results did not reach statistical significance (P = 0.189). Likewise, the Mean total LOS before initiation of treatment was 12.18 ± 12.80 days compared to 6.45 ± 10.99 days after, although the results also did not reach statistical significance (P 0.274).
At the 12 month mark, there was a significant difference in the distribution of change in hemoglobin level among the three treatment groups (P=0.008) (Table 2). Specifically, when compared to baseline hemoglobin, patients on voxelotor alone had a greater increase in hemoglobin than those on crizanlizumab alone (0.90 ± 1.19 g/dl vs -0.17 ± 0.56 g/dl, P = 0.008). Similarly, when compared to baseline hemoglobin, patients on both crizanlizumab and voxelotor had a greater increase in hemoglobin than those on crizanlizumab alone (0.70 ± 1.03 g/dl vs -0.17 ± 0.56 g/dl, P = 0.010).
Conclusion This study suggests that patients treated with both crizanlizumab and voxelotor had an improvement in number of VOC related hospitalizations, LOS and hemoglobin level but our results could not achieve statistical significance due to our small sample size. Further real-world studies are needed to further understand the effects of combination therapies on acute care utilization.
Disclosures
Andemariam:Hemanext: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aruvant: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk A/S: Consultancy, Membership on an entity's Board of Directors or advisory committees; Terumo BCT: Consultancy, Membership on an entity's Board of Directors or advisory committees; Emmaus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Shenox: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal