Abstract
Introduction Pediatricians treating newly diagnosed severe hemophilia A patients are confronted with the challenge to protect the children against bleeds that are potentially life-threatening or have long-term damaging effects on joints. A well-established protective effect has early prophylaxis with coagulation factor VIII (FVIII). However, higher doses and "peak treatment moments” have been associated with a rate of inhibitor formation against factor VIII in up to 40%1,2. Another still controversial topic is the impact of the product choice on inhibitor risk. There is some evidence that plasma-derived factor VIII, or certain concentrates, are less immunogenic3,4. We report our experience with an individualized approach to managing PUPs at a single hemophilia treatment center (HTC) in Germany.
Methods PUPs with severe hemophilia A (FVIII:C <1%) treated at the Gerinnungszentrum Rhein-Ruhr (GZRR), Germany, between January 2013 and July 2022 were followed prospectively.
Our treatment approach includes a thorough training of parents to early detect signs of bleeds and avoid injuries with special focus on the first 50 exposure days (EDs). Patients are treated with a human plasma-derived FVIII (pdFVIII/VWF) concentrate for at least the first 50 EDs with an individually tailored treatment schedule that leads to a full prophylactic schedule. Initial doses are chosen low, given weekly or every 10 days and escalated according to bleeding tendency. In case of more severe bleeds, high doses of 60-80 IU/kg are chosen to avoid treatment for more than 2 days. Minor bleeds or hematomas are not always treated.
The parents stay in close contact with the center so that the decision to treat with pdFVIII/VWF can be done on a case-by-case basis.
We monitor joint health by prophylactic physiotherapy and regular ultrasound every 3-6 months. Non-urgent surgical procedures are postponed, and venous access system implants are avoided within the first 100 EDs.
Inhibitor levels are measured using the modified Bethesda assay every 3-4 EDs until ED 100, and every 3 months thereafter for 2 years.
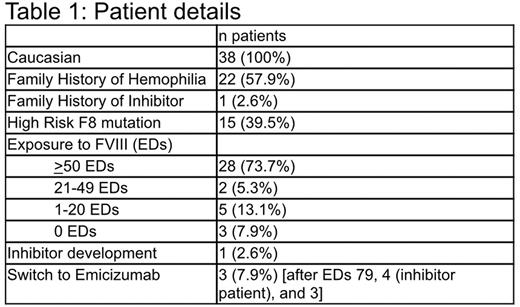
Results Data from 38 consecutive PUPs with severe hemophilia A were collected, of which 35 (92.1%) have been treated with FVIII.
Of the 38 patients, 34 started regular treatments within the first 10 EDs. The schedule was tailored individually ranging from 21 IU/kg every 10 days up to 40 IU/kg twice per week. One patient was treated on-demand for an intracranial bleed from ED 1-100.
Only 3 spontaneous bleeds in 3 patients were treated; those bleeds had led to diagnosis. All other patients remained free of spontaneous bleeds requiring treatment.
One patient developed a high titer inhibitor that was diagnosed at ED 4. He had received 35 IU/kg for treatment of a hematoma and 2 further equal doses in 2 separate weeks. The mother had reported unusual hematoma formation with a dark and more extended middle part following a slow resolution already before the Bethesda assay was positive.
Discussion Our approach to managing PUPs includes treatment with human pdFVIII/VWF for at least the first 50 EDs. During this time, we treat bleeds only when considered necessary but then with high doses to minimize further infusions. We report 1 inhibitor development in a prospective cohort of 38 patients. In contrast, between 2003 and 2012, four of nine (44%) PUPs treated at our center developed inhibitors during the first 20 EDs (2 low-, 2 high-titer inhibitors). At that time, we used recombinant FVIII (rFVIII) for prophylaxis in most patients and were less selective about which and how to treat bleeds with FVIII.
After at least 50 EDs we usually discuss the option to switch patients from pdFVIII/VWF to rFVIII as the injection volume is generally lower.
Conclusion With a rate of 1/30 (3.3%) patients with more than 20 EDs developing an inhibitor, these data support the use of our individualized management approach allowing for a tolerization phase with FVIII on the way to a full prophylactic schedule. We consider the close contact and thorough training of the parents/guardians crucial in order to closely monitor the patients and assure adequate protection from bleeds.
1. Gouw SC et al. J Thromb Haemost 2007; 5(7): 1383-90.
2. Gouw SC et al. Blood 2013; 121(20): 4046-55.
3. Klukowska A, et al. Haemophilia 2018; 24(2): 221-8.
4. Peyvandi F et al. A. New Engl J Med 2016; 374(21): 2054-64.
Disclosures
Reinhardt:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cerus: Membership on an entity's Board of Directors or advisory committees; Medac: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal