Abstract

Introduction Coagulopathy is a well-recognized complication of COVID-19, with an incidence of thrombotic events as high as 31% in patients who are critically ill from COVID-19 infection. Estrogen-containing hormonal therapies are also well known to increase the risk of thrombosis. There have been no studies evaluating if estrogen-containing hormonal therapies potentiate thrombotic risk during COVID-19 infection, and there is no consensus on the clinical role of holding hormonal therapy during infection. We performed a large retrospective cohort study to evaluate the thrombotic risk of estrogen therapy during COVID-19 infection in both pre-menopausal and post-menopausal patients.
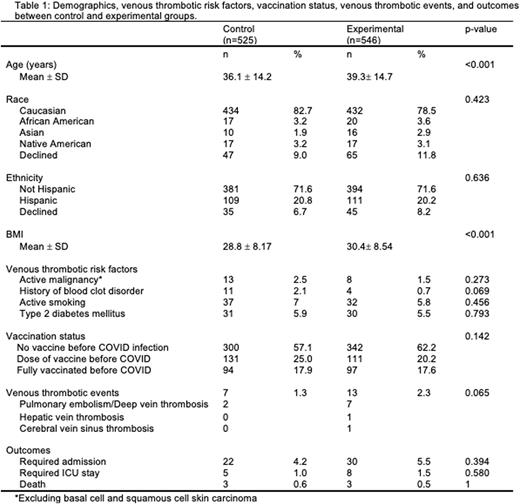
Methods To assess the association between hormonal therapies and thrombosis in COVID-19 patients, we collected data on all female Oregon Health & Science University patients aged 18-80 years who tested positive for COVID-19 between 12/2020 and 2/2022 and were on hormonal therapies. We included a comprehensive range of hormonal therapies to ensure an accurate representation of both pre-menopausal and post-menopausal patients. The experimental group consisted of patients on combined hormonal contraceptives and hormone replacement therapy via the oral and transdermal routes, which have all been associated with increased thrombotic risk. The control group consisted of patients with a progesterone intrauterine device or on topical estrogen, neither of which is associated with an increased thrombosis risk. Pregnant individuals and patients on therapeutic anticoagulation before testing positive for COVID-19 were excluded. We collected data on demographics, vaccination status, thrombotic risk factors, venous thrombotic events, and outcomes (Table 1). The continuous variables were not normally distributed, and p-values were calculated using the Mann-Whitney test. The p-values for the categorical variables were calculated using Fisher's exact test. We then performed cox proportional hazards regression analysis to assess the thrombotic risks between control and experimental groups, while controlling for potential confounding variables. Venous thrombotic events were defined as pulmonary embolism (PE), deep vein thrombosis (DVT), cerebral vein sinus thrombosis (CVST), or hepatic vein thrombosis that occurred within 6 months of testing positive for COVID-19.
Results In total, 1071 individuals met inclusion criteria. The experimental group included 546 patients, 407 on combined hormonal contraceptives (CHC) and 139 on oral or transdermal hormone replacement therapy (HRT). The control group included 525 patients, 410 with a progesterone intrauterine device and 115 using topical estrogen creams. The control and experimental groups were similar in all demographics and thrombotic risk factors, except for age (P<0.001) and body mass index (BMI) (p<0.001). There were no differences between groups in the rate of hospitalization for COVID-19, ICU stay for COVID-19, or death. There were 2 venous thrombotic events with an event rate of 1.3% in the control group and 9 events with a rate of 2.3% in the experimental group (p= 0.065). All of these events occurred within 2 months of COVID-19 positive test results. Increasing age (p<0.001) was significantly associated with a higher venous thrombosis risk. A cox proportional hazards regression analysis was utilized to evaluate the hazards ratio (HR) of venous thrombosis between groups, controlling for age and BMI. Patients in the experimental group had a HR of 5.76 (CI 1.24-26.77, p=0.026) for venous thrombotic events. In this model, the HR was 1.06 for age (CI 1.02-1.11, p=0.003) and 1.05 for BMI (CI 1.01-1.10, p=0.018).
Conclusion Overall, this data suggests that estrogen-containing oral and transdermal hormonal therapies increase the risk of thrombosis in COVID-19 positive patients, although absolute risks were small. Thrombotic risk was also associated with increasing age and higher BMI. Based on these findings, providers may consider temporarily discontinuing hormonal therapies in patients with COVID-19 who are at high risk for thrombosis, especially older patients, those with a higher BMI, and those on higher doses of oral estrogen.
Disclosures
Shatzel:Aronora, Inc.: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal