Abstract
Background: Targeted protein degradation (TPD) is attracting significant interest given the success of early protein degraders, e.g., lenalidomide and pomalidomide, and TPD's potential advantages over other therapeutic approaches. SP-3164, an oral, next-generation molecular glue, is currently in IND-enabling studies and is expected to enter the clinic in 2023. SP-3164 interacts with the cereblon (CRBN) component of a CRL4 E3 ligase, inducing recruitment of select proteins, i.e., Ikaros (IKZF1) and Aiolos (IKZF3), leading to their ubiquitination and proteasomal degradation. To date, most CRBN-binding compounds, including SP-3164, contain a chiral aminoglutarimide moiety and exist as (S)- and (R)-enantiomers. Studies have shown that in many of these CRBN-binding compounds, it is the (S)-enantiomer that binds to CRBN with increased affinity and thus may be the primary species resulting in the desired protein degradation and anti-cancer activity. However, the (S)- and (R)-enantiomers can chemically interconvert, preventing development of a single enantiomer. SP-3164 was designed using deuterium to stabilize the (S)-enantiomer with the potential for an improved therapeutic profile compared to the clinically studied racemate, avadomide. We previously demonstrated that compared to avadomide, SP-3164 shows increased binding affinity to CRBN, while the deuterium-stabilized (R)-enantiomer (SP-3165) does not bind CRBN at clinically relevant concentrations. Here, we further characterize SP-3164 pharmacological properties compared to avadomide, SP-3165, and lenalidomide, and demonstrate its activity in preclinical lymphoma models.
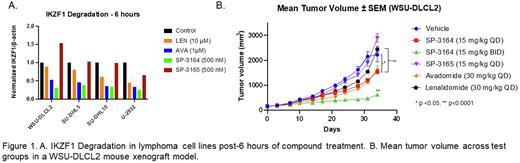
Results: In addition to CRBN binding, efficiency of TPD is another critical parameter. In in vitro studies of diffuse large B cell lymphoma (DLBCL) cell lines of different subtypes (WSU-DLCL2, SU-DHL-5, SU-DHL-10 and U-2932), SP-3164 (500 nM) resulted in greater IKZF1 degradation at 6 hours (Figure 1A), when compared to avadomide (AVA, 1 µM) or lenalidomide (LEN, 10 µM). By contrast, SP-3165 (500 nM) did not result in meaningful IKZF1 depletion, further corroborating the (R)-enantiomer as the inactive species.
Pharmacokinetics (PK) of SP-3164, SP-3165, and avadomide were studied in mice. Oral administration of either SP-3164 or SP-3165 resulted in increased exposure to the dosed enantiomer when compared to an equivalent dose of avadomide, and minimal exposure to the other enantiomer, while the elimination half-life of SP-3164 and SP-3165 was approximately half that of the enantiomers of avadomide. Therefore, SP-3164 has the potential to be administered at lower doses in clinic and provide a more flexible dosing regimen compared to avadomide, while still maintaining sufficient exposure for anticancer activity.
The in vivo efficacy of SP-3164 for lymphoma was studied in a WSU-DLCL2 mouse xenograft model (Figure 1B). Compared to lenalidomide, SP-3164 exhibited significantly increased tumor growth inhibition (TGI). SP-3164 and avadomide showed similar activity at comparable doses and dosing regimens (SP-3164, 15 mg/kg QD, and avadomide, 30 mg/kg QD). SP-3165 lacked significant anti-cancer effects indicating that the (R)-enantiomer does not contribute to the direct anti-cancer activity of avadomide, as anticipated based on its lack of binding to CRBN. Due to the shorter elimination half-life of SP-3164 compared to avadomide, SP-3164 was also dosed BID. The SP-3164 BID treated group had the largest TGI, suggesting that the in vivo anti-cancer activity may be due to sustained exposure of SP-3164 at the tumor resulting in prolonged reduction of target protein levels.
Conclusion: SP-3164 is a novel, CRBN-binding molecular glue with potential attractive therapeutic properties compared to the racemate, avadomide, and to lenalidomide. SP-3164 showed increased IKZF1 degradation efficiency compared to other molecular glues and significant anti-cancer activity as a monotherapy in an in vivo lymphoma mouse xenograft model. Future studies will evaluate SP-3164 efficacy and direct potential combinations to support clinical development of SP-3164 in DLBCL and other non-Hodgkin's lymphomas.
Disclosures
Santiesteban:Salarius Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Duncan:Salarius Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Mirza:Salarius Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Jacques:Salarius Pharmaceuticals, Inc.: Consultancy; DeuteRx, LLC: Current Employment, Current equity holder in private company; Neuromity Therapeutics, Inc: Current equity holder in private company; Zywie, LLC: Consultancy; Poxel SA: Consultancy, Current equity holder in publicly-traded company. DeWitt:Salarius Pharmaceuticals, Inc.: Consultancy; DeuteRx, LLC: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Poxel SA: Consultancy, Current equity holder in publicly-traded company; Neuromity Therapeutics, Inc.: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; RIFFIT, Inc: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Enveric Biosciences, Inc.: Consultancy; Revere Pharmaceuticals, Inc.: Current equity holder in private company. Iyer:Seagen: Consultancy, Research Funding; Spectrum: Research Funding; Merck: Research Funding; Rhizen: Research Funding; Legend: Research Funding; Affimed: Research Funding; Salarius Pharmaceuticals, Inc.: Consultancy; Innate: Research Funding; Yingli: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; CureBio: Honoraria; Target Oncology: Consultancy, Honoraria; Myeloid: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal