Abstract
Introduction: To investigate the demographics and treatment details of the acute myeloid leukemia (AML) patients who were diagnosed and followed up in Turkey.
Methods: Patients who were recorded on the database of Turkish AML Registry project were included in this study retrospectively if they were diagnosed before 1st of Jan 2022. Demographics, patient, and disease related parameters both at the time of diagnosis and at the follow up and treatment outcomes were presented.
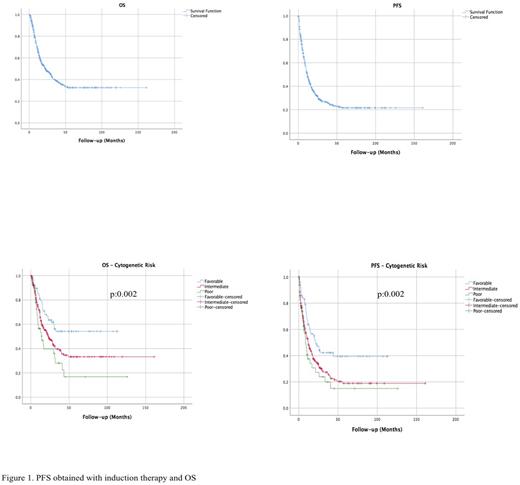
Results: A total of 519 patients were included in the study, median follow-up period was 14.0 (SD:25.6) months and 44.5% of the patients were female. Median age at the time of diagnosis was 54 (18-89). According to the FAB classification most common subtypes were M4 (24.9%), M2 (24.6%), M5 (16.8%) and M1 (14.2%) and AML, NOS (60.2%) was the most common type according to the WHO classification. Of the patients 64.7%, 24.7%, 14.7%, was intermediate, favorable and poor respectively considering AML risk stratification (ELN 2017) by genetics. Only 4.6% of the patients had extramedullary involvement at the time of diagnosis. For the first induction therapy, 89.0% of the patients had received intensive regimens composing anthracyclines and ARA-C, the rate of intensive therapies declined to 41.2% at the time of first relapse. ARA-C (69.6%) backbone and Azacitidine alone (6.9%) were the most commonly used regimens and 13.2% underwent allo-HCT at first remission. Response rates obtained by the induction therapies were as follows; 68.9% CR (including 11.9% MLFS), 11.9% PR, 8.1% SD, 11.1% PD, the ratio was 24.7% for CR2. Median overall survival was not reached for the favorable risk group, were 22.8 months for the intermediate risk and 14.3 months for the poor risk group (p:0.002) and 22.5 months for the entire population. Median progression free survival were 21.5 months for the favorable risk group, 11.9 months for the intermediate risk and 8.6 months for the poor risk group (p:0.003) and 11.5 months for the entire population (Figure 1).
Conclusion: Progression free survival obtained after induction therapy and overall survival were relatively shorter than the ones which were presented by other real-world registries. Lack of early access to the targeted and novel agents, like Flt3 inhibitors, Venetoclax, IDH1 and 2 inhibitors, CPX351 and Glasdegib should be a potential explanation to this relatively short PFS and OS. We have also been aware of un-ideal access to well established and nation wide cytogenetic laboratory service to induce early integration of targeted agents to the treatment and better risk stratification to determine the patients who should obtain the best prolonged survival via allo-HCT.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal