Abstract
Introduction Recent studies have demonstrated that Blinatumomab is more effective and less toxic than intensive chemotherapy in children and young persons (CYP) with relapsed B-ALL. Although Blinatumomab is standard of care in adults with persistent MRD during first line therapy based on the BLAST study, there is no published evidence in CYP of its efficacy and toxicity in this setting. We report our experience of Blinatumomab as a toxicity sparing alternative to chemotherapy in first line treatment of CYP with B-ALL.
Patients and Methods We audited the outcome of patients treated according to a consensus UK guideline for first line treatment with Blinatumomab of CYP patients with B-ALL who were considered unfit for post-remission intensive chemotherapy or CR1 HSCT because of toxicity to previous chemotherapy or significant co-morbidities. Treating clinicians obtained consent for treatment with Blinatumomab as standard of care after counselling patients and carers about the risk and benefits of this approach. The guideline mandated discussion of all such patients at national tumour board meetings and data was captured through a referral proforma and follow-up questionnaire. It recommended replacing consolidation and/or interim maintenance phases with 2 cycles of blinatumomab as a single agent with a 1-2 week break between cycles. Patients who obtained an MRD response (defined as MRD <0.01%) received delayed intensification (DI) followed by maintenance, or maintenance therapy alone if they were unfit for DI. Patients who had persistent MRD >0.01% were categorised as Blinatumomab failures and received either further chemotherapy, HSCT or CART depending on performance status. Events were defined as death, relapse, or secondary cancer.
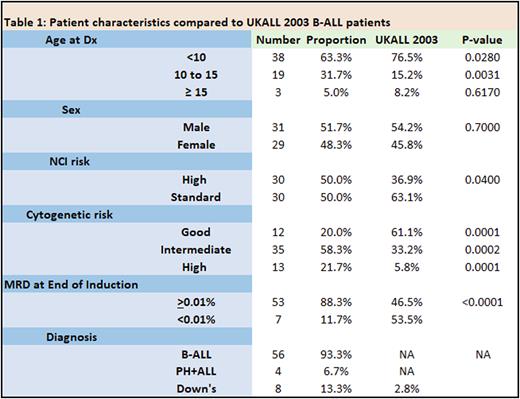
Results Sixty patients were eligible for analysis, including 4 cases of Ph+ ALL and 8 with Down syndrome. Compared to the B-ALL cohort in a previous trial, UKALL 2003, there was a higher proportion of NCI high risk, cytogenetic high risk, end of induction MRD positive and Down Syndrome patients (Table 1). All patients were in morphological complete remission prior to receiving Blinatumomab. Blinatumomab was administered for toxicity (47/60 (78%), including G3/4 pancreatitis, typhlitis, severe infections often requiring intensive care or surgery, disseminated fungal infections, CNS haemorrhage or liver failure) or co-morbidities (Down syndrome, Li-Fraumeni syndrome (n=2), Schimke immune-osseous dysplasia and 7q11.21-23 duplication) or in one case to minimise transfusion requirements (Jehovah's witness.) In the remaining 13 cases, blinatumomab was either administered for end of consolidation MRD of ≥0.05% (n=10) in patients unfit for intensive chemotherapy/HSCT, or positive end of induction MRD in patients with HR cytogenetics (n=3). Blinatumomab replaced consolidation and interim maintenance therapy in 32/60 (53%) cases, and interim maintenance in the remaining 28/60 (47%) cases. Blinatumomab was well tolerated with 1 G3 neurotoxicity (seizure in a Down syndrome patient who had not received prophylactic anti-convulsants) and no grade 2-4 CRS. Of the 50/60 patients with positive MRD pre-Blinatumomab (median = 0.1%, range = 0.01%-8%), 48 (96%) had a reduction in level to <0.01% (45 after cycle 1, 3 after cycle 2), and 10 patients maintained MRD negativity before and after 2 cycles of Blinatumomab. In the two patients who did not respond (3%), 1 received CART followed by transplant for persistent MRD, the other is receiving intensive chemotherapy. Of the responders, 47 (80%) received DI and maintenance and 10 maintenance alone. With a median follow up of 16 months (range 2-44 months), 2 patients have relapsed (1 at 2 years, the other at 9 months) but remain alive. The 4 patients with Ph+ ALL and the 8 with Down syndrome are alive and in remission, with a median follow up of 12.5 months (range 7-22 months).
Conclusion In this large case series, we found Blinatumomab is safe and highly active with promising short-term efficacy in a higher risk cohort of CYP patients with B-ALL in first remission. Our results support its use in place of intensive chemotherapy for patients with relative chemo-intolerance. Furthermore, if the efficacy is sustained on longer follow-up, the results argue for replacing parts of post-remission intensive chemotherapy with Blinatumomab to reduce attendant morbidity and mortality.
Disclosures
Moorman:Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal