Abstract
Introduction Peripheral T cell lymphomas are heterogeneous neoplasms of post thymic T cells with distinct clinical, pathological and genetic characteristics. We previously identified the NPM1-TYK2 fusion in patients with CD30+ lymphoproliferative disorders, including 15% of primary cutaneous ALK− anaplastic large cell lymphoma. The oncogenicity of NPM1-TYK2 and its effect on T cell differentiation, the molecular mechanisms and signaling pathways through which it drives lymphomagenesis invivo remain unknown.
Methods To investigate the effect of NPM1-TYK2 on oncogenesis and T cell differentiation, we established a conditional transgenic model (CD4-NPM1-TYK2). To investigate cooperativity with Trp53, CD4-NPM1-TYK2 mice were crossed with Trp53 floxed mice to generate CD4ΔTrp53-NPM1-TYK2 mice with T cell-specific concomitant heterozygous loss of Trp53. Mice (n = 15 per genotype) were monitored over time for tumor development. Hematopoietic organs were harvested and subjected to histolopathological and immunophenotypic analysis. Serum cytokine and immunoglobulin profiles were analyzed by Luminex multiplex immunoassays. The effects of NPM1-TYK2 on the physiological function of T cells were examined by growth curves and cell cycle analysis using anti-CD3/CD28 stimulated CD4+ T cells from CD4-NPM1-TYK2 mice in vitro. To evaluate the signaling sequences of NPM1-TYK2 expression, unbiased mass spectrometry (MS)-driven phosphoproteomics was performed. The pharmacological effect of Deucravacitinib, an allosteric TYK2 inhibitor, on NPM1-TYK2+ murine T cells and human T cell lymphoma-derived cells were examined.
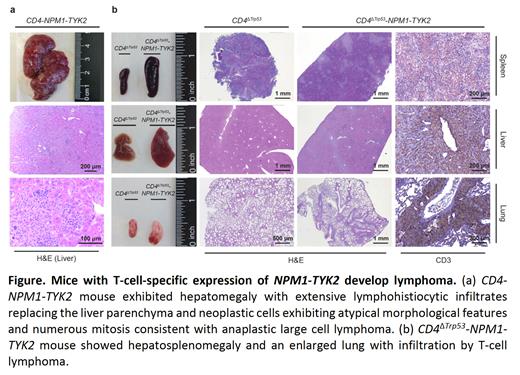
ResultsCD4-NPM1-TYK2 mice showed significant splenomegaly (n = 20; 104.8 mg vs 86.5 mg) and developed lymphoma with hepatomegaly due to atypical neoplastic infiltrates. Notably, CD4ΔTrp53-NPM1-TYK2 mice (n = 5; 15.4 months) exhibited an earlier onset of lymphoma compared to CD4-NPM1-TYK2 mice (n = 4; 19.8 months). Immunophenotypic characterization of CD4+ T cells from the spleen of CD4-NPM1-TYK2 mice (n = 7) revealed an increase in effector T cells (CD44+/CD62L−, 51.5% vs 39.4%) and a decrease in naïve T cells (CD44−/CD62L+, 32.9% vs 44.8%), suggesting NPM1-TYK2 promotes effector T cell differentiation in vivo. Among all T helper cell subtypes, increased Tfh cells was observed in CD4-NPM1-TYK2 mice (n = 8; ICOS+/CXCR5+/PD1+/BCL6+, 27.4% vs 15.5%), indicating NPM1-TYK2 mediates the commitment of Tfh cell skewing in vivo. Serum cytokine profile of CD4-NPM1-TYK2 mice also showed elevated Tfh-related cytokines (n = 4; IL-1β, IL-4, IL-6, IL-10, IL-12). Tfh cells are crucial for the formation of germinal center (GC) B cells and their differentiation into plasma cells. Elevated expansion of GC B cells (n = 5; CD95+/PNA+, 4.8% vs 3.8%) was also observed. Increased plasma cells (B220−/CD138+, 9.1% vs 3.8%) and elevated immunoglobulins (n = 6; IgA, IgG2b) in the serum corroborated observed lymphoplasmacytic cells in the histology of CD4-NPM1-TYK2 mice.
Among MS-identified phosphoproteins demonstrating the YX(2-5)L motif of JAK family kinase substrates, we observed elevated levels of tyrosine phosphopeptides implicating STAT1/3/5 activation. Western blotting of CD4+ T cells from CD4-NPM1-TYK2 mice revealed hyperphosphorylation of STAT1/3/5. NPM1-TYK2+ T cells (n = 3 per genotype) were hyperreactive to stimulation in vitro, displaying increased activated cells (CD69+/CD25+, 83/80% vs 76/73%). The growth curves of NPM1-TYK2+ T cells showed increased growth (2.6-fold) with more proliferating cells in the S phase of cell cycle (31% vs 25%) whilst control CD4+ T cells stopped proliferating 10 days post-stimulation. Intriguingly, the NPM1-TYK2-mediated growth-promoting effect was attenuated by Deucravacitinib.
Conclusion Conditional transgenic expression of NPM1-TYK2 in mature T-cell lineage promotes Tfh cell differentiation accompanied by intense GC B cell and plasma cell expansion, and hepatosplenomegaly with disseminated lymphoma accelerated by concomitant heterozygous ablation of Trp53in vivo. The biologic effects of NPM1-TYK2 expression are propagated through STAT1/3/5 activation. These data provide novel insights on the signaling, cellular immunologic and oncogenic consequences of NPM1-TYK2 expression in CD4+ T cells. Our studies implicate NPM1-TYK2 as a de facto oncogene in vivo and provides a valuable pre-clinical model for precision medicine.
Disclosures
Lim:EUSA Pharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal