Key Points
ITP women do not increase their risk of severe bleeding during pregnancy.
NITP is associated with NITP history and the severity of maternal ITP during pregnancy.
Abstract
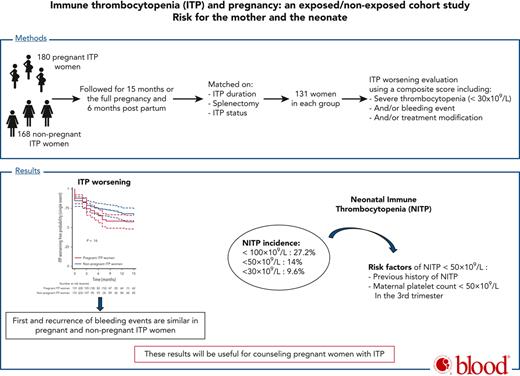
The risk of immune thrombocytopenia (ITP) worsening during pregnancy and neonatal ITP (NITP) have never been prospectively studied. We included 180 pregnant and 168 nonpregnant women with ITP in a prospective, multicenter, observational cohort study. A total of 131 pregnant women with ITP were matched to 131 nonpregnant women with ITP by history of splenectomy, ITP status (no response, response, complete response), and duration. Groups were followed for 15 months. The primary outcome was the first occurrence of ITP worsening defined by a composite end point including bleeding events and/or severe thrombocytopenia (<30 × 109/L) and/or ITP treatment modification. We also studied the recurrence of ITP worsening and the incidence of NITP and risk factors. The first occurrence of ITP worsening did not differ between pregnant and nonpregnant women with ITP (53.4 per 100 person-years [95% confidence interval {CI}, 40.8-69.9] vs 37.1 [95% CI, 27.5-50.0]; hazard ratio {HR}, 1.35 [95% CI, 0.89-2.03], P = .16). Pregnant women with ITP were more likely to have recurrence of severe thrombocytopenia and treatment modification (HR, 2.71 [95% CI, 1.41-5.23], P = .003; HR, 2.01 [95% CI, 1.14-3.57], P = .017, respectively). However, recurrence of severe bleeding events was not different between groups (P = .4). Nineteen (14%) neonates showed NITP <50 × 109/L. By multivariable analysis, NITP was associated with a previous offspring with NITP and maternal platelet count <50 × 109/L within 3 months before delivery (adjusted odds ratio, 5.55 [95% CI, 1.72-17.89], P = .004 and 4.07 [95% CI, 1.41-11.73], P = .009). To conclude, women with ITP do not increase their risk of severe bleeding during pregnancy. NITP is associated with NITP history and the severity of maternal ITP during pregnancy. These results will be useful for counseling women with ITP.
Introduction
Adult immune thrombocytopenia (ITP) is an autoimmune disease characterized by acquired thrombocytopenia with no clinically apparent associated condition or other clear cause of thrombocytopenia. It can affect people of all ages, with a higher prevalence in women when diagnosed during the childbearing period. Retrospective cohort studies suggested that pregnancy in women with ITP might be linked to decreased platelet count requiring the initiation of treatment or therapeutic modification in about 15% to 30% of patients. However, an appropriate nonpregnant ITP comparative group is lacking, and clinical relevance of the decreased platelet count is uncertain. The overall prognosis seems good.1-10 Neonatal ITP (NITP), presumably due to passive transfer of maternal antiplatelet antibodies, may occur, with an incidence of 15% to 30%, but the incidence of severe bleeding in neonates and risk factors of NITP are not well known.1,8,11-13 Several risk factors have been reported, but with the exception of a previous sibling with NITP, no other risks have been consistent among studies.
ITP presents a challenge during pregnancy for both the mother and neonate, and current guidelines for pregnant women with ITP are based mostly on expert opinion and results of retrospective studies. The main goal of our study was to assess whether pregnancy was associated with ITP worsening regarding decreased platelet count, bleeding events, and treatment modification. Secondary objectives were to study whether pregnancy was associated with obstetrical complications, the incidence of NITP, and risk factors for NITP.
Patients and methods
Study design
A total of 32 centers from the French ITP reference center network participated in this nationwide prospective, multicenter, observational, exposed–nonexposed cohort study (ClinicalTrials.gov: NCT02892630).
Patients
Over a 5-year period, from 2013 to 2018, we enrolled women with a diagnosis of primary ITP according to the international consensus criteria.14 Exposed women were pregnant, 18 years or older, with a pregestational diagnosis of ITP. Nonexposed women had ITP, were of childbearing age, and were nulligravid or at 12 months’ post-partum stage from a previous pregnancy.
Follow-up
Exposed and nonexposed women were followed for 15 months (±3 months), which corresponds to the full term of the pregnancy plus 6 months after delivery for pregnant women.
Matching
Pregnant and nonpregnant women who were followed for the full period (15 ± 3 months) were matched based on 3 criteria: history of splenectomy, ITP duration (ie, persistent [<1 year] or chronic), and ITP status at inclusion. We chose splenectomy on the basis of retrospective data suggesting that it could be a risk factor of NITP and ITP worsening.4 The ITP status (no response, response, complete response) was used because it is an international criterion to describe ITP severity.14 Finally, we included ITP duration (persistent vs chronic) because it has been demonstrated that ITP can heal spontaneously during the first year.15
ITP status was classified according to the international consensus as follows: nonresponse (NR), with platelet count less than 30 × 109/L or the need for therapeutic modification in the previous 8 weeks regardless of platelet count; remission/response (R), with platelet count between 30 × 109/L and 100 × 109/L without treatment or with treatment unchanged in the last 8 weeks; and complete remission/response (CR), with platelet count 100 × 109/L or more without treatment or with treatment unchanged in the last 8 weeks.14 Women lost to follow-up were not included in the matching.
This study was approved by the institutional review board and ethics committee of Bicêtre hospital (PP13-013, 15/05/2013) and was performed in accordance with the Declaration of Helsinki. Written information was delivered by the investigators and oral consent was obtained from the patient in accordance with French law for noninterventional study.
Study end points
The primary end point was the first occurrence of ITP worsening during pregnancy and the 6-month postpartum period for exposed women and during the 15-month follow-up for nonexposed women. ITP worsening was assessed with a composite score, defined by at least one of the following: (1) occurrence of a new bleeding event, and/or (2) occurrence of severe thrombocytopenia (ie, platelet count less than 30 × 109/L), and/or (3) ITP treatment initiation or modification except for ITP treatment to prepare for delivery. Treatment to prepare for delivery was defined by a treatment made of steroids and/or intravenous immunoglobulin (IVIg) exclusively prescribed by the doctor to prepare for delivery up to 4 weeks before the expected due date.
We also performed an analysis of recurrence of ITP worsening.
Secondary end points were each criterion of the main composite end point, pregnancy and birth outcomes in women with ITP, including preterm delivery; occurrence of gestational diabetes or other medical complications during pregnancy; occurrence of obstetrical complications including perinatal bleeding; and incidence as well as risk factors for NITP. For NITP, an available nadir platelet value for the neonate between day 1 and 8 was considered. NITP was defined as mild with neonate platelet count 50 to 100 × 109/L, moderate with 30 to 50 × 109/L, and severe with less than 30 × 109/L.
Data collection
The schedule of visits during the follow-up was left to the clinician’s judgment/discretion, but at least 5 visits for the pregnant women with ITP and 2 visits for the nonpregnant women with ITP were recommended.
An electronic standardized case report form (Cleanweb, Telemedicine, France) was used to collect data on age, ITP diagnosis, pregnancy, platelet count, new bleeding manifestations at each visit, and treatment initiation or intensification. To uniformly assess the severity of bleeding events, each center used a bleeding score widely used for adult ITP in France and previously described by our group (supplemental Table 1, available on the Blood website).16 Bleeding severity is graded from 1 to 19 based on clinical evaluation, decreased hemoglobin level, and requirement for blood or platelet transfusion. Because all of our patients were of childbearing age, age from the original scale was not considered. Severe bleeding was defined by a score greater than 8.
Sample size
The sample size was based on the expected frequency of ITP worsening in the pregnant group (exposed) and the ability to detect a difference of the main end point between the pregnant and nonpregnant group. On the basis of a 20% expected frequency, a 2-sided significance level of .05, and a power of 80%, we planned to include a sample of 150 pregnant women to obtain a precision of estimation of 6%. A comparative group of 1:1 with 150 nonpregnant women enabled us to detect a difference of ITP worsening of 12% (8% vs 20%) with a bilateral 5% alpha risk and a power of 80%.
Baseline characteristics analysis
Baseline categorical data are expressed as number and percentage [n (%)] and continuous data as median [interquartile range (IQR)]. Baseline characteristics of matched exposed and nonexposed groups were compared by using a mixed-effects logistic regression model with pair as a random intercept.
Main analysis
The main end point and each criterion of the composite end point were expressed as incidence rates and 95% confidence intervals (CIs) according to the Kaplan-Meier method. Time to event was the time from inclusion to the first event among the 3 criteria of the composite end point. Survival curves are displayed according to group (exposed vs nonexposed). Each criterion of the composite end point was accordingly analyzed. We compared groups by using a Cox model with shared frailty to take into account the matching nature of the data. We also analyzed the occurrence of multiple events of ITP worsening and each criterion of the composite end point by using the Anderson-Gill Cox model. Participants lost to follow-up were censored at the last clinical visit.
The secondary end points are described with number and percentage for categorical variables, except for the occurrence of a bleeding event, severe bleeding event, severe thrombocytopenia, and treatment modification. Continuous secondary end points are expressed as median (IQR).
The association of maternal features and history of NITP during previous pregnancies with NITP in the current pregnancy (ie, neonatal platelet count less than 50 × 109/L and 30 × 109/L) was assessed with a univariable logistic regression model. Unadjusted odds ratios (ORs) and 95% CIs were estimated. Variables with a P value less than .2 were selected for multivariable analyses, and adjusted ORs (aORs) and 95% CIs were estimated. Interactions were tested in bivariate models.
All tests were 2-tailed, and P value less than .05 was considered statistically significant. We did not use multiple imputation. Analyses involved using STATA v15.0 (StataCorp, College Station, TX).
Results
Patient characteristics
We included 348 adult women with persistent or chronic ITP, including 180 pregnant women and 168 nonpregnant women (Figure 1). The numbers of patients included per year and per center are detailed in supplemental Table 2. Nine pregnant women with ITP were lost to follow-up before delivery; for 6, the follow-up stopped after a miscarriage. The remaining 171 pregnant women with ITP were followed until delivery. Their demographics and ITP characteristics are detailed in Table 1. Among women followed for the full follow-up (158 pregnant women and 156 nonpregnant women) (Figure 1), we matched 131 pregnant women with ITP to 131 nonpregnant women with ITP on history of splenectomy, ITP status and ITP duration. The comparison of demographics and ITP characteristics of the 131 paired women and the 171 pregnant women with ITP followed until delivery are detailed in Table 1. During follow-up, pregnant women with ITP and nonpregnant women with ITP had a median (IQR) number of medical visits of 10 (7-12) and 5 (3-7), respectively.
Enrollment and outcomes. ∗Taken into account for frequency of miscarriage when it was the reason for the woman being lost to follow-up; n = 6/9 lost to follow-up after a miscarriage. In the other cases, reasons for the woman being lost to follow up were that she moved or was not seen at follow-up consultations or contacted by telephone. ITP, immune thrombocytopenia.
Enrollment and outcomes. ∗Taken into account for frequency of miscarriage when it was the reason for the woman being lost to follow-up; n = 6/9 lost to follow-up after a miscarriage. In the other cases, reasons for the woman being lost to follow up were that she moved or was not seen at follow-up consultations or contacted by telephone. ITP, immune thrombocytopenia.
Demographics and immune thrombocytopenia characteristics
| . | Pregnant women with ITP followed up to delivery (n = 171) . | Matched pregnant women with ITP, exposed (n = 131) . | Matched nonpregnant women with ITP, non-exposed (n = 131) . | P Value∗ . |
|---|---|---|---|---|
| Age at ITP diagnosis, y, median (IQR) | 24.1 (18.4, 28.1) | 24.2 (19.4, 28.1) | 23.7 (18.0, 30.8) | .38 |
| Age at enrollment, y, median (IQR) | 30.4 (26.9, 34.5) | 30.4 (26.7, 34.9) | 31.4 (24.4, 37.9) | .45 |
| Age at pregnancy diagnosis, y, median (IQR) | 30.1 (26.9, 34.1) | 30.4 (26.4, 34.6) | ||
| Women with at least 1 prior pregnancy | 116 (67.8) | 90 (68.7) | ||
| Women with at least 1 prior pregnancy after ITP diagnosis (n = 115/89) | 81 (70.4) | 62 (69.7) | ||
| Neonatal thrombocytopenia occuring at previous pregnancy after ITP diagnosis (n = 78/61) | 21 (26.9) | 14 (23) | ||
| ITP previous treatment | ||||
| No treatment | 60 (35.1) | 47 (35.9) | 33 (25.2) | .06 |
| If yes, no. of lines, median (IQR) | 2 (1-3) | 2 (1-3) | 2 (2-3) | .92 |
| Splenectomy | 22 (12.9) | 17 (13) | 17 (13) | 1 |
| ITP duration at pregnancy diagnosis (pregnant women with ITP) or at time of inclusion (nonpregnant women with ITP) | 1 | |||
| Persistent | 10 (5.8) | 9 (6.9) | 9 (6.9) | |
| Chronic | 161 (94.2) | 122 (93.1) | 122 (93.1) | |
| ITP status at pregnancy diagnosis or at time of inclusion (controls) | 1 | |||
| CR | 85 (49.7) | 50 (38.2) | 50 (38.2) | |
| R | 69 (40.4) | 64 (48.8) | 64 (48.8) | |
| NR | 17 (9.9) | 17 (13.0) | 17 (13.0) |
| . | Pregnant women with ITP followed up to delivery (n = 171) . | Matched pregnant women with ITP, exposed (n = 131) . | Matched nonpregnant women with ITP, non-exposed (n = 131) . | P Value∗ . |
|---|---|---|---|---|
| Age at ITP diagnosis, y, median (IQR) | 24.1 (18.4, 28.1) | 24.2 (19.4, 28.1) | 23.7 (18.0, 30.8) | .38 |
| Age at enrollment, y, median (IQR) | 30.4 (26.9, 34.5) | 30.4 (26.7, 34.9) | 31.4 (24.4, 37.9) | .45 |
| Age at pregnancy diagnosis, y, median (IQR) | 30.1 (26.9, 34.1) | 30.4 (26.4, 34.6) | ||
| Women with at least 1 prior pregnancy | 116 (67.8) | 90 (68.7) | ||
| Women with at least 1 prior pregnancy after ITP diagnosis (n = 115/89) | 81 (70.4) | 62 (69.7) | ||
| Neonatal thrombocytopenia occuring at previous pregnancy after ITP diagnosis (n = 78/61) | 21 (26.9) | 14 (23) | ||
| ITP previous treatment | ||||
| No treatment | 60 (35.1) | 47 (35.9) | 33 (25.2) | .06 |
| If yes, no. of lines, median (IQR) | 2 (1-3) | 2 (1-3) | 2 (2-3) | .92 |
| Splenectomy | 22 (12.9) | 17 (13) | 17 (13) | 1 |
| ITP duration at pregnancy diagnosis (pregnant women with ITP) or at time of inclusion (nonpregnant women with ITP) | 1 | |||
| Persistent | 10 (5.8) | 9 (6.9) | 9 (6.9) | |
| Chronic | 161 (94.2) | 122 (93.1) | 122 (93.1) | |
| ITP status at pregnancy diagnosis or at time of inclusion (controls) | 1 | |||
| CR | 85 (49.7) | 50 (38.2) | 50 (38.2) | |
| R | 69 (40.4) | 64 (48.8) | 64 (48.8) | |
| NR | 17 (9.9) | 17 (13.0) | 17 (13.0) |
All results are given as number of events (%) unless otherwise specified. n is specified only when data are missing.
CR, complete response; IQR, interquartile range; ITP, immune thrombocytopenia; NR, nonresponse; R, response.
Mixed logistic regression model.
Pregnancy and ITP outcomes
Pregnancy and ITP outcomes of the 171 pregnant women followed until delivery are described in Table 2. Among this group, 131 were matched.
Immune thrombocytopenia (ITP) and pregnancy outcome for 171 women with pre-existing ITP and their neonates
| . | Total (n = 171)∗ . |
|---|---|
| ITP outcome | |
| Bleeding event during pregnancy except postpartum hemorrhage | 36 (21.1) |
| Treatment intensification or initiation during pregnancy | 52 (30.4) |
| Treatment to prepare delivery | 67 (39.2) |
| CT only | 32 (47.8) |
| IVIg only | 21 (31.3) |
| CT+IVIg | 11 (16.4) |
| Other | 3 (4.5) |
| Pregnancy outcome | |
| Single pregnancy | 165 (97.1) |
| Miscarriage/stillbirth | 9 (5.2) |
| Platelet count at delivery, median × 109/L (IQR) (n = 158) | 109.5 (83-154) |
| <50 × 109/L | 9 (5.7) |
| <70 × 109/L | 27 (17.1) |
| ≥70 × 109/L | 131 (82.9) |
| Delivery (n = 169) | |
| Vaginal delivery | 130 (76.9) |
| Cesarean delivery | 39 (23.1) |
| Obstetrical reason independent of ITP | 29 (74.4) |
| Because of ITP | 6 (15.4) |
| Other reason | 4 (10.2) |
| Preterm delivery (before 37 weeks) | 16 (9.3) |
| Moderate preterm (between 36 and 32 weeks) | 12 (7.0) |
| Very preterm (between 28 and 32 weeks) | 4 (2.3) |
| Epidural anesthesia (n = 158) | |
| Yes | 109 (69.0) |
| If no, reason (n =41) | |
| ITP reason | 19 (46.3) |
| Refusal | 10 (24.4) |
| Obstetrical reason | 3 (7.3) |
| Other reasons | 9 (22.0) |
| If yes, platelet count <70 × 109/L | 2 (1.9) |
| Postpartum hemorrhage (n = 166) | 16 (9.6) |
| Platelet count, median × 109/L (IQR) | 94 (78-117) |
| Neonate outcome | |
| Newborn weight, g, median (IQR) (n = 154) | 3167.5 (2850-3470) |
| Neonatal thrombocytopenia (<100 × 109/L) (n = 136) | 37 (27.2) |
| <50 × 109/L | 19 (14.0) |
| <30 × 109/L | 13 (9.6) |
| Hemorrhage complication | 2 (1.2) |
| If yes, platelet count, median × 109/L (IQR) | 6.5 (6-7) |
| IVIg treatment | 18 (10.5) |
| If yes, platelet count, median × 109/L (IQR) | 25.5 (15-35) |
| Platelet transfusion | 8 (4.7) |
| If yes, platelet count, median × 109/L (IQR) | 15 (12-31) |
| . | Total (n = 171)∗ . |
|---|---|
| ITP outcome | |
| Bleeding event during pregnancy except postpartum hemorrhage | 36 (21.1) |
| Treatment intensification or initiation during pregnancy | 52 (30.4) |
| Treatment to prepare delivery | 67 (39.2) |
| CT only | 32 (47.8) |
| IVIg only | 21 (31.3) |
| CT+IVIg | 11 (16.4) |
| Other | 3 (4.5) |
| Pregnancy outcome | |
| Single pregnancy | 165 (97.1) |
| Miscarriage/stillbirth | 9 (5.2) |
| Platelet count at delivery, median × 109/L (IQR) (n = 158) | 109.5 (83-154) |
| <50 × 109/L | 9 (5.7) |
| <70 × 109/L | 27 (17.1) |
| ≥70 × 109/L | 131 (82.9) |
| Delivery (n = 169) | |
| Vaginal delivery | 130 (76.9) |
| Cesarean delivery | 39 (23.1) |
| Obstetrical reason independent of ITP | 29 (74.4) |
| Because of ITP | 6 (15.4) |
| Other reason | 4 (10.2) |
| Preterm delivery (before 37 weeks) | 16 (9.3) |
| Moderate preterm (between 36 and 32 weeks) | 12 (7.0) |
| Very preterm (between 28 and 32 weeks) | 4 (2.3) |
| Epidural anesthesia (n = 158) | |
| Yes | 109 (69.0) |
| If no, reason (n =41) | |
| ITP reason | 19 (46.3) |
| Refusal | 10 (24.4) |
| Obstetrical reason | 3 (7.3) |
| Other reasons | 9 (22.0) |
| If yes, platelet count <70 × 109/L | 2 (1.9) |
| Postpartum hemorrhage (n = 166) | 16 (9.6) |
| Platelet count, median × 109/L (IQR) | 94 (78-117) |
| Neonate outcome | |
| Newborn weight, g, median (IQR) (n = 154) | 3167.5 (2850-3470) |
| Neonatal thrombocytopenia (<100 × 109/L) (n = 136) | 37 (27.2) |
| <50 × 109/L | 19 (14.0) |
| <30 × 109/L | 13 (9.6) |
| Hemorrhage complication | 2 (1.2) |
| If yes, platelet count, median × 109/L (IQR) | 6.5 (6-7) |
| IVIg treatment | 18 (10.5) |
| If yes, platelet count, median × 109/L (IQR) | 25.5 (15-35) |
| Platelet transfusion | 8 (4.7) |
| If yes, platelet count, median × 109/L (IQR) | 15 (12-31) |
All results are given as number of events (%) unless otherwise specified.
CT, corticosteroids; IQR, interquartile range; IVIg, intravenous immunoglobulin.
n = 171 unless otherwise specified.
The 131 matched pregnant and nonpregnant women with ITP did not differ in ITP worsening when considering the first event (53.4 per 100 person-years [95% CI, 40.8-69.9] vs 37.1 per 100 person-years [27.5-50.0]; hazard ratio [HR], 1.35 [95% CI, 0.89-2.03], P = .16) (Figure 2). Pregnant and nonpregnant women with ITP did not differ in incidence of severe thrombocytopenia (ie, platelet count less than 30 × 109/L) (28.5 per 100 person-years [20.7-39.4] vs 25.0 per 100 person-years [17.8-35.2]; HR, 1.13 [95% CI, 0.71-1.82], P = .61) or incidence of bleeding events (21.1 per 100 person-years [14.8-30.2] vs 13.5 per 100 person-years [8.7-20.9]; HR, 1.54 [95% CI, 0.87-2.73], P = .13), and when considering severe bleeding only (13.2 per 100 person-years [8.5-20.4] vs 7.8 per 100 person-years [4.4-13.7]; HR, 1.71 [95% CI, 0.83-3.49], P = .14) (Figure 2; supplemental Figure 1). However, ITP treatment modification including ITP treatment initiation (except for ITP treatment used to prepare to delivery) was more frequent for pregnant than for nonpregnant women with ITP (31.9 per 100 person-years [23.6-43.2] vs 18.6 [12.7-27.1]; HR, 1.73 [95% CI, 1.06-2.82], P = .03) (Figure 2). The median (IQR) platelet count nadir within the month before ITP treatment change was similar in pregnant and nonpregnant women with ITP (16 × 109/L [7-26] and 17 × 109/L [6-27]).
Incidence of first immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. First ITP worsening was evaluated by a composite end point, including first event of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. First event and multiple events were assessed. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. ∗Except treatment to prepare delivery.
Incidence of first immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. First ITP worsening was evaluated by a composite end point, including first event of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. First event and multiple events were assessed. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. ∗Except treatment to prepare delivery.
In the exposed group, ITP worsened in 24.5%, 32.5%, 39.5%, and 13.5% of women in the first, second and third trimester and in the postpartum period, respectively.
When we looked at the 171 pregnant women followed until delivery (Table 2), results were comparable with those of the 131 paired pregnant women in term of bleeding events, severe thrombocytopenia, and treatment modification. Among them, 67 women (39.2%) received treatment in preparation for delivery [without any other ITP treatment during pregnancy for 19 (28.4%)], for almost all (64 of 67, 95.5%) based on corticosteroids and/or IVIg. Three women received another treatment, namely, platelet transfusion for 2 women and romiplostin for 1 woman.
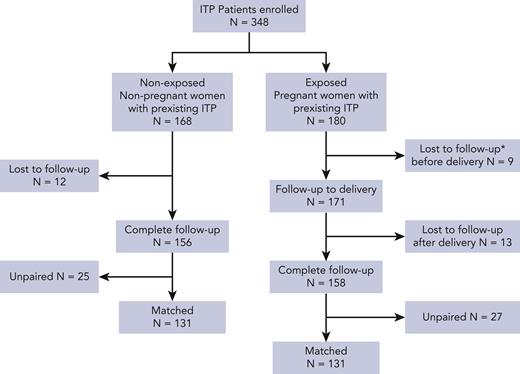
We also studied the potential for multiple events for our matched 131 pregnant and nonpregnant women. Pregnant women were more likely than nonpregnant women to have multiple events regarding severe thrombocytopenia and treatment modification (HR, 2.71 [95% CI, 1.41-5.23], P = .003; HR, 2.01 [95% CI, 1.14-3.57], P = .017 respectively) (Figure 3). However the groups were similar in regard to the recurrence of bleeding events (HR, 1.83 [95% CI, 0.91-3.65], P = .09) and severe bleeding events (HR 1.38 [95% CI, 0.6-2.9]; P = .4) (Figure 3; supplemental Figure 1).
Incidence of recurrent immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. ITP worsening was evaluated by a composite end point including recurrences of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. ∗Except treatment to prepare delivery.
Incidence of recurrent immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. ITP worsening was evaluated by a composite end point including recurrences of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. ∗Except treatment to prepare delivery.
ITP worsening was exactly the same in the 2 matched groups when comparing ITP status before pregnancy to 6 months after delivery for pregnant women with ITP and at inclusion to 12 to 15 months later for nonpregnant women with ITP (16.8% in both groups, P = .57).
Obstetrical complications
Nine (5%) miscarriages/stillbirths occurred in the 180 pregnant women enrolled in the study, with 4 (2%) early miscarriages (less than 13 weeks’ gestation), 3 (1.6%) late miscarriages (14-24 weeks), 1 (0.5%) fetal death, and 1 (0.5%) stillbirth (Table 2). The risk of miscarriage reported in the general population in France is about 12%.17 Among the 171 pregnant women with ITP followed to delivery, 8 (4.6%) exhibited gestational diabetes, 2 (1.1%) pre-eclampsia, and 1 woman (0.5%) severe hypertension. No case of thrombosis occurred.
The risk of gestational diabetes reported in Europe is about 5%.18
Delivery complications
Overall, 16 of the 171 (9.3%) women with ITP included had a preterm delivery, defined as delivery before 37 weeks’ gestation, including 12 (7%) moderate or late preterm (32-36 weeks) and 4 (2.3%) very preterm (28-32 weeks) deliveries (Table 2). The rate of preterm delivery reported in Europe is from 5% to 10%.19,20 Maternal platelet count at delivery was more than 70 × 109/L for 131 (82.9%) women with ITP. Cesarean delivery was performed in 39 (23.1%) women. The investigators reported ITP as the “main reason” for 6 (15%) cesarean deliveries. A total of 109 (69%) women had epidural analgesia, including 2 with a platelet count less than 70 × 109/L (with no complications). Sixteen of 166 (9.6%) women experienced postpartum hemorrhage, with the lowest platelet count at 50 × 109/L. Blood loss data were available for 6 of these women; blood loss was more than 1000 mL for 2 women. Two patients required red blood cell transfusion, associated with platelet transfusion in 1 patient, and a third patient received platelet transfusion only.
Impact of ITP on neonates
Among the 177 neonates who were born from 171 mothers with ITP followed until delivery, data collection was incomplete for 6 neonates, and finally data for 171 neonates were analyzed. Table 2 shows the incidence, treatment, and complications of NITP. Platelet count was available for 136 neonates. In total, 37 (27.2%) newborns exhibited NITP: 18 (13.2%), 6 (4.4%), and 13 (9.6%) exhibiting mild, moderate, and severe NITP, respectively. IVIg was administered to 18 (10.5%) newborns; their median (IQR) platelet count was 25.5 × 109/L (6-56). Eight newborns with a median platelet count of 13.5 × 109/L (6-50) received platelet transfusion. NITP was complicated by a bleeding event in 2 newborns, with platelet count less than 10 × 109/L in both. One neonate died from intracranial hemorrhage that was diagnosed in utero. The mother’s blood sample was screened for presence of GPIIbIIIa antibody by using an indirect monoclonal antibody immobilization of platelet antigens test, which was negative. No other tests were performed to rule out alloimmune thrombocytopenia. The second neonate had a minor bleeding event (bleeding score = 1) with a favorable evolution after treatment with IVIg and platelet transfusions.
On multivariable analysis, mother’s previous history of NITP and maternal platelet count less than 50 × 109/L within 3 months before delivery were independently associated with moderate or severe NITP (ie, neonate platelet count less than 50 × 109/L) (adjusted OR, 5.55; 95% CI, 1.72-17.89; P = .004 and 4.07; 1.41-11.73; P = .009) (Table 3). Also, maternal platelet count less than 50 × 109/L within 3 months before delivery was the sole predictor of severe NITP (ie, neonate platelet count less than 30 × 109/L) (OR, 6.15; 95% CI, 1.72-21.95; P = .005) (supplemental Table 3). Among the 21 women with a history of NITP, nadir platelet count data during these previous pregnancies were available for 17 of the 21 neonates with a median (IQR) platelet count of 31 × 109/L (15-60). A new episode of NITP was observed for 9 (42.8%) current pregnancies with a median platelet count of 31 × 109/L (17-38).
Factors associated with neonatal thrombocytopenia <50 × 109/L in 171 newborns
| . | Neonatal thrombocytopenia (platelet count <50 × 109/L) . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|---|
| Total(n = 136) . | No(n = 117) . | Yes(n = 19) . | Unadjusted odds ratio [95% CI] . | P value∗ . | Adjusted odds ratio [95% CI] . | P value† . | |
| Maternal platelet count at delivery < 50 × 109/L (n=127/109/18) | 8 (6.3) | 6 (5.5) | 2 (11.1) | 2.15 [0.40-11.57] | .37 | ||
| Maternal platelet count < 50 × 109/L within 3 months before delivery | 44 (32.4) | 33 (28.2) | 11 (57.9) | 3.5 [1.29-9.47] | .01 | 4.07 [1.41-11.73] | .009 |
| Disease status at pregnancy diagnosis | .14 | ||||||
| CR | 62 (45.6) | 57 (48.7) | 5 (26.3) | 1 (ref) | |||
| R | 59 (43.4) | 49 (41.9) | 10 (52.6) | 2.33 [0.74-7.27] | |||
| NR | 15 (11.0) | 11 (9.4) | 4 (21.1) | 4.15 [0.96-17.93] | |||
| Disease status worsening between M0 and M9 | 65 (47.8) | 52 (44.4) | 13 (68.4) | 2.71 [0.96-7.61] | .06 | ||
| Previous history of neonatal thrombocytopenia | 20 (14.7) | 13 (11.1) | 7 (36.8) | 4.67 [1.56-13.96] | .006 | 5.55 [1.72-17.89] | .004 |
| Previous splenectomy | 16 (11.8) | 13 (11.1) | 3 (15.8) | 1.50 [0.38-5.85] | .56 | ||
| Treatment to prepare delivery IVIg and/or CT | 58 (42.7) | 47 (40.2) | 11 (57.9) | 2.05 [0.77-5.47] | .15 | ||
| . | Neonatal thrombocytopenia (platelet count <50 × 109/L) . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|---|
| Total(n = 136) . | No(n = 117) . | Yes(n = 19) . | Unadjusted odds ratio [95% CI] . | P value∗ . | Adjusted odds ratio [95% CI] . | P value† . | |
| Maternal platelet count at delivery < 50 × 109/L (n=127/109/18) | 8 (6.3) | 6 (5.5) | 2 (11.1) | 2.15 [0.40-11.57] | .37 | ||
| Maternal platelet count < 50 × 109/L within 3 months before delivery | 44 (32.4) | 33 (28.2) | 11 (57.9) | 3.5 [1.29-9.47] | .01 | 4.07 [1.41-11.73] | .009 |
| Disease status at pregnancy diagnosis | .14 | ||||||
| CR | 62 (45.6) | 57 (48.7) | 5 (26.3) | 1 (ref) | |||
| R | 59 (43.4) | 49 (41.9) | 10 (52.6) | 2.33 [0.74-7.27] | |||
| NR | 15 (11.0) | 11 (9.4) | 4 (21.1) | 4.15 [0.96-17.93] | |||
| Disease status worsening between M0 and M9 | 65 (47.8) | 52 (44.4) | 13 (68.4) | 2.71 [0.96-7.61] | .06 | ||
| Previous history of neonatal thrombocytopenia | 20 (14.7) | 13 (11.1) | 7 (36.8) | 4.67 [1.56-13.96] | .006 | 5.55 [1.72-17.89] | .004 |
| Previous splenectomy | 16 (11.8) | 13 (11.1) | 3 (15.8) | 1.50 [0.38-5.85] | .56 | ||
| Treatment to prepare delivery IVIg and/or CT | 58 (42.7) | 47 (40.2) | 11 (57.9) | 2.05 [0.77-5.47] | .15 | ||
The results are given as number of events (%).
CI, confidence interval; CR, complete response; CT, corticosteroids; IVIg, intravenous immunoglobulin; NR, nonresponse; R, response; ref, reference category.
Wald test from univariable logistic regression.
Multivariable logistic regression model, adjusted for maternal platelet count and previous history of neonatal thrombocytopenia.
Discussion
Pregnancy can be a source of anxiety for women with ITP and for their physicians. Indeed, published retrospective studies have suggested a risk of ITP worsening of more than 30% for the mother and risk of thrombocytopenia in the newborn, with conflicting data on maternal risk factors for NITP. This prospective exposed/nonexposed cohort study performed in this setting shows that women with ITP do not increase their risk of severe bleeding during pregnancy. The study gives useful results for counseling women with ITP.
According to our primary outcome based on the first ITP worsening event, defined as a composite end point including the first occurrence of new bleeding, severe thrombocytopenia, or ITP treatment initiation/modification, we did not observe significant ITP worsening during pregnancy. The first occurrence of severe thrombocytopenia or bleeding events was similar between matched pregnant and nonpregnant women with ITP. We cannot exclude a potential confounder of gestational thrombocytopenia because, in healthy women, pregnancy shifts the normal bell-shaped distribution of platelet counts downward by 25 to 50 × 109/L at the time of pregnancy.21 However, it is unlikely that gestational thrombocytopenia can explain the occurrence of severe thrombocytopenia below 30 × 109/L. Treatment was more frequent during pregnancy but without any clinical or biological evidence of worsening. In contrast, ITP worsening based on recurrence of events was more frequent in pregnant than in nonpregnant women with ITP, with more treatment modification and more severe thrombocytopenia. However, the 2 groups did not differ in bleeding events, in particular severe bleeding events, which is the more relevant clinical event, as was observed for the first event. According to international and French guidelines,22,23 for most pregnant women receiving treatment for their ITP during pregnancy, the platelet count was less than 30 × 109/L. These more frequent ITP treatment modification and recurrence of severe thrombocytopenia in the pregnant than in the nonpregnant group may have been due in part to closer monitoring during pregnancy, as shown by the more frequent medical visits in the pregnant than in the nonpregnant group. The design of our study does not allow for concluding whether the increase in treatment intervention is beneficial, but the treatment may have prevented hemorrhagic complications and disease progression during pregnancy. ITP treatments administered during pregnancy were almost exclusively based on corticosteroids and IVIg and were well tolerated, with a low incidence of diabetes and hypertension and no episode of thrombosis.
Exposed and nonexposed women with ITP were matched on history of splenectomy, because previous retrospective studies suggested that splenectomy per se could be a predictor of ITP worsening, disease status based on international criteria,14 and ITP duration at inclusion. These criteria might not capture a representative ITP history at the individual level. According to this limitation, we observed a tendency for more treatment-naive women in the exposed than in the nonexposed group. However, the number of previous ITP therapeutic lines and ITP duration were similar between the groups.
In the late postpartum period, the risk of ITP worsening was low and seemed to be linked to the natural course of the disease. At the end of the study, the incidence of ITP status worsening as compared with ITP status at inclusion was low and exactly the same in both pregnant and nonpregnant groups (16.8%). This reassuring result confirms that most of the pregnant women with ITP would return to their previous ITP status within months after delivery, and that pregnancy is not associated with risk of further disease worsening.
We did not compare the evolution of the pregnancy in women with ITP with a control group of pregnant women without ITP. However, in light of data from French and European registries, our results do not suggest any increased risk of obstetrical or delivery complications in pregnant women with a pre-gestational diagnosis of ITP. The risk of gestational diabetes, miscarriage, and rate of preterm delivery were similar to those expected in the general population of pregnant women.17-20,24,25 We do not confirm the retrospective data of Belkin et al., who reported that ITP could be associated with preterm delivery before 34 weeks’ gestation and with increased perinatal mortality.26
The rate of NITP incidence that we observed was similar to that described previously,1,8,11-13 with severe NITP occurring in 9.6% of pregnant women with ITP. From retrospective studies, risk factors for NITP were previous pregnancy with NITP11-13,27,28 and history of splenectomy.4,8,28-30 We do not confirm the association between splenectomy and NITP, considering that only 22 (12.8%) pregnant women underwent splenectomy. As previously described, risk of NITP on multivariable analysis was associated with history of an older sibling with NITP. However, the risk of mild or severe NITP during a subsequent pregnancy was only 42%. Except for rare studies,12,29 most studies and international guidelines suggested that ITP severity in the mother, including platelet count, was not associated with risk of NITP. Our study clearly shows the opposite, and severity of ITP in the mother with a platelet count of less than 50 × 109/L in the third trimester should be considered a risk factor for the occurrence of severe NITP. Although NITP has not been found to be prevented by ITP treatment intensification in the mother, physicians should be aware of this risk.28,30
There are limitations to our study. Regarding our primary end point, we planned for 150 women in each group. After matching, only 131 women remained in each group. From our results, it seems unlikely that we could have missed a difference in severe thrombocytopenia, but we cannot exclude a slight trend of increase in bleeding events missed due to lack of power. Besides, we could not perform matching per center because the number of exposed and nonexposed women was not homogeneously distributed between the centers. However, it is unlikely that this nonhomogeneous inclusion was a source of bias because each center belongs to the French ITP reference center network and follows the same national guidelines for ITP care.23 Another potential limitation was our composite end point, which combines objective criteria for example, bleeding events and severe thrombocytopenia, and criteria depending on clinician practice as treatment intervention. However, to allow a full assessment of the complexity of ITP worsening, it seems important not to limit our evaluation to bleeding events even though they were the more relevant clinical events for physicians.31 Another limitation of our study is the absence of systematic testing for anti-platelet antibodies in pregnant women, which did not allow for studying the possible link between the presence of these antibodies and the risk of NITP. Similarly, antiplatelet alloantibodies were not tested in thrombocytopenic neonates, nor were platelet phenotypes analyzed in the parents, and we cannot exclude that the death observed in the severely thrombocytopenic neonate was not due to fetomaternal platelet antigen incompatibility.
To conclude, our study, based on a large prospective exposed–nonexposed cohort, shows that women with ITP do not increase their risk of severe bleeding during pregnancy. Nearly 85% of the women showed a return to their ITP status after pregnancy. This is strictly similar to the change in ITP status observed among nonpregnant women with ITP followed during the same observation period, which confirms that the prognosis for the mother is excellent. Severe NITP was not uncommon and, as previously shown, was associated with NITP in a previous pregnancy. Our study demonstrates that the severity of ITP during pregnancy should now also be considered a risk factor. Nevertheless, women with ITP should not be discouraged if they want to become pregnant, because the prognosis for the newborn is reassuring when therapeutic measures proposed in international guidelines are observed. These findings will be useful for counseling women with ITP about the impact of pregnancy on their own health and that of their offspring.
Acknowledgments
We thank Francoise Roudot-Thoraval for helping design study, Laura Smales for her careful reading and editing of the manuscript, Aurelien Sokal for help in making the figures, and Didier Bouscary, Deau Fischer, Gaelle Guettrot-Imbert, Vassilis Tsastaris, Veronique Le Guern and France Teillet-Thiebaud for patient recruitment.
This work was supported by grants (PHRC; AOM12206) from the French health ministry. The sponsor was Assistance Publique - Hôpitaux de Paris (délégation à la Recherche Clinique et à l'Innovation).
Authorship
Contribution: B.G. and V.L. designed the study and initiated this work; S.G., B.G., M. Mahevas, M. Michel, V.L., and F.C.-P. wrote the report; and all authors made substantial contributions to acquisition of data, revised the article critically and gave final approval of the manuscript to be submitted.
Conflict-of-interest disclosure: B.G. serves as expert for AMGEN, Novartis, Grifols, Sobi, and Borhinger. M. Mahevas received funds for research from GSK, and received fees from LFB. M. Michel received consultancy fees from Amgen, Novartis, and Argenx. D.G. received consultancy fees from Novartis and Shire Takeda. The remaining authors declare no competing financial interests.
Correspondence: Bertrand Godeau, Service de Médecine Interne, 51 avenue du Maréchal de Lattre de Tassigny, Créteil, France, 94000; e-mail: bertrand.godeau@aphp.fr.
References
Author notes
For original data, please contact bertrand.godeau@aphp.fr.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Incidence of first immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. First ITP worsening was evaluated by a composite end point, including first event of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. First event and multiple events were assessed. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of a Cox model with shared frailty. ∗Except treatment to prepare delivery.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/1/10.1182_blood.2022017277/5/m_blood_bld-2022-017277-gr2.jpeg?Expires=1768012565&Signature=X~qAHy-aBh599OySE~9f0lwLha-S0NpzC-MD9ghlDcavxT5Mn99w2AKwQp8HC7GLsFW-e1lCHmuJtrvktwLT8PlQuS8tRvGYX~-82sfLNczvBmhWd1iVUVHnByDWsFplP-TeyY5zqjwsLEznkemkm9v6-aK3cEV4irV9vMlreYdFVBcHnZY4pGYOOC3NKgP1ZeCx4Hj02kplyWqF0Qkv~QaD9N6CHej4bAfxj~jLhwOr5qUcfSjOPfmfO4JFJp0BDTBibCXzkUQnUVLad0d-bv03C3~M4rwRJQpwuuCYEmJFsjUM0YzZZNbNdf91TPFUe56fLQevCAsBL8kwXm7rFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Incidence of recurrent immune thrombocytopenia (ITP) worsening during pregnancy among matched pregnant and nonpregnant women with ITP. ITP worsening was evaluated by a composite end point including recurrences of severe thrombocytopenia <30 × 109/L and/or bleeding event and/or treatment modification/initiation. (A) ITP worsening-free probability (and 95% confidence interval [CI]) was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. (B-D) Free probability of severe thrombocytopenia (platelet count <30 × 109/L), bleeding event, and treatment modification/initiation (and 95% CI), respectively, was estimated with the Kaplan-Meier method and compared in the 2 groups with the use of an Anderson-Gill Cox model. ∗Except treatment to prepare delivery.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/1/10.1182_blood.2022017277/5/m_blood_bld-2022-017277-gr3.jpeg?Expires=1768012565&Signature=loWvaocM89EqxhjsTQfPeX9qpbyldbS9bwFQllmYlaVv9X8vwt4Li5QkduRhvHMpJMvcYDPFJizboZ8QOQHLyIHkOfEDjJdWjm~7uC9RK0FXGYj3p-lqDpbvpQi6SoUKBU7JlrRCQEC0j-LkRD8NNVFHLKT1t5RlNlqIU3WhE4NpeRIYsZS6e9JEOuI~vAONgTE0PRfimMEOHY7zRvvjDicNJl7h5PskVVg2V~xTNAt3jOK1D9R7q9WIlJA0-ljkokDeUp3Bv1GN8r~XHVjapp7m3UvyL6AkvXcXGqdCPJlpBFinMesWLIuzNz~OXXYdhsdP3nvVG1SJP2-ToKe~tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal