In this issue of Blood, Tischer et al1 add to the growing pool of evidence that allostery within von Willebrand factor (VWF) regulates platelet adhesion.
Rapid platelet adhesion to vascular injuries has long been known to depend on VWF, a flexible multimeric protein with a structure that underlies its ability to target platelets. A balance between intramolecular interactions and hemodynamic forces keeps plasma VWF from binding circulating platelets. Upon immobilization, VWF unravels and elongates in response to fluid flow, which activates specific monomers within the multimer for platelet adhesion.2 The molecular mechanisms that govern this highly regulated switch have been a subject of intense investigation.
Distinct domains with specific functions comprise a single monomer of a VWF multimer. The platelet-binding interface and the scissile bond for the metalloprotease ADAMTS13 (von Willebrand factor-cleaving protease), are located in the VWF A1 and A2 domains, respectively. Efforts to elucidate the mechanisms by which VWF binds to its platelet receptor, glycoprotein Ibα (GPIbα), and is proteolyzed in response to hemodynamic forces have focused largely on the individual domains’ direct interactions with their counterparts.3 However, a growing body of literature indicates that allosteric mechanisms control A1-GPIbα and A2-ADAMTS13 interactions. Flexible polypeptides flanking the A1 domain act as gatekeepers to the interface for GPIbα.4 Furthermore, interactions between the VWF A1 and A2 domains may additionally regulate VWF–platelet interaction, as well as VWF proteolysis by ADAMTS13.5,6
All but one of VWF’s domains (A2) contain a long-range disulfide bond(s) that constrains their overall architecture and inhibits extensive unfolding when tensioned at their N- and C-termini by physiological forces.3 The tertiary structure of the VWF A2 domain is instead supported by noncovalent interactions that permit unwinding upon stimulation. Among its multiple structural features, the A2 domain contains vicinal cysteines (C1669 and C1670) that form a disulfide bond that reversibly integrates with the hydrophobic, β-sheet core to help stabilize A2’s folded conformation, a key structural feature in shielding the scissile bond from ADAMTS13.3 A2 expressed as a polypeptide without C1669 and C1670 (G1481-R1668) inhibits platelet adhesion to immobilized VWF, possibly due to its affinity for A1’s GPIbα-binding conformation.7 In plasma, C1669 and C1670 are found in multiple redox states, with the nonoxidized forms (ie, loss of disulfide) reported to result in diminished VWF–platelet interaction.5
In contrast to the findings in these previous reports, Tischer et al found that the absence of a disulfide bond between C1669 and C1670 resulted in a slightly but significantly increased affinity between GPIbα and a model VWF polypeptide spanning its 3 A domains.1 The overall platelet adhesion profile was elevated, with no change in the characteristic shear-dependent catch-slip behavior for the polypeptide with substitutions, C1669S and C1670S, suggesting that the mechanical pathway in A1 for GPIbα-binding remained intact. Hydrogen–deuterium exchange mass spectrometry detected greater solvent access to not only the A2 domain for C1669S/C1670S but also secondary structures in A1 that form part of VWF’s interface with GPIbα. Thermodynamic analyses suggested that the more dynamic conformation of A2, due to the C1669S and C1670S substitutions, lessens A1–A2 interaction in the model VWF polypeptide, possibly giving A1 a greater opportunity for GPIbα-binding. Together, these data suggest that A1 uncoupling from A2 may be an initiating step for exposing tensioned VWF to platelets. Intriguingly, kinetic binding assays demonstrated that an interaction between GPIbα and A2 (with and without C1669 and C1670), expressed in Escherichia coli but not mammalian cells, inhibited the binding of GPIbα to A1, providing an alternative interpretation of previous reports that concluded that E. coli–derived A2 (with or without C1669 and C1670) binds A1 to inhibit GPIbα-binding.5,7
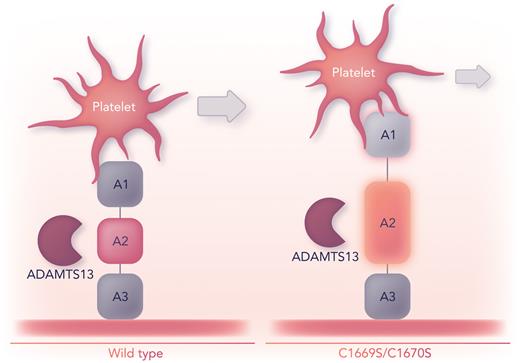
How unfolding of A2 due to a loss of its vicinal disulfide bond may manifest as multimeric VWF in vivo remains to be determined. Multimeric VWF with C1669A and C1670A is more susceptible to proteolysis by ADAMTS13, consistent with a more dynamic A2 conformation.8 With a greater chance for A1–GPIbα interactions due to a loss of the vicinal disulfide bond, enhanced VWF proteolysis can be posited as a result of heightened rheological sensitivity for A2 unfolding in VWF-platelet complexes (see figure).9 Although this postulate would portend a loss of high–molecular weight VWF, missense variants at C1669 or C1670 have not been associated with von Willebrand disease.
Intramolecular interaction(s) between A1 and A2 regulates VWF shear-dependent functions. The absence of the C1669–C1670 disulfide bond in the VWF A2 domain of a model polypeptide (A1A2A3) leads to greater deformations that promote intramolecular dissociation between the A1 and A2 domains. As a result, platelets translocate more slowly across immobilized A1A2A3C1669S/C1670S than they do across A1A2A3wt. Prolonged tension from bound platelets under flow and a more easily unfolded A2 should enhance VWF’s susceptibility to proteolysis by ADAMTS13. Professional illustration by Somersault18:24.
Intramolecular interaction(s) between A1 and A2 regulates VWF shear-dependent functions. The absence of the C1669–C1670 disulfide bond in the VWF A2 domain of a model polypeptide (A1A2A3) leads to greater deformations that promote intramolecular dissociation between the A1 and A2 domains. As a result, platelets translocate more slowly across immobilized A1A2A3C1669S/C1670S than they do across A1A2A3wt. Prolonged tension from bound platelets under flow and a more easily unfolded A2 should enhance VWF’s susceptibility to proteolysis by ADAMTS13. Professional illustration by Somersault18:24.
The vicinal cysteines in A2 are highly conserved, suggesting that the A1–A2 interaction may be a common mechanism for regulating VWF–platelet interactions. However, whether and to what extent these vicinal cysteines form a disulfide bond in other species remain undetermined. Also, how species with no cysteines in their A2 domain (eg, Danio rerio, Oryzias latipes, and Takifugu rubripes) regulate VWF structure to maintain hemostasis is unclear.
Despite reaching opposing conclusions, Tischer et al and Butera et al point to a cooperation between A1 and A2 in which C1669 and C1670 are key residues for regulating platelet adhesion.1,5 Should this disulfide be specifically broken (all 169 cysteines within a monomer of mature VWF are assigned to a disulfide bond3), the different fates of the functional groups (eg, thiol, sulfenic acid, or their derivatives) and their ramifications would also be important to consider. As demonstrated for transaldolase, cysteines can covalently bind lysines via a sulfur–oxygen–nitrogen bridge that acts as an allosteric redox switch.10 As new insights emerge from this continuing debate, new opportunities to identify novel targets in VWF to promote hemostasis in bleeding disorders or to abrogate platelet adhesion in thrombotic disorders will arise.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal