In this issue of Blood, Hakkarainen and an international team of clinical investigators provide an in-depth analysis of 52 patients with excision repair cross-complementation group 6 like 2 (ERCC6L2) syndrome caused by germline biallelic ERCC6L2 mutations.1 Their findings offer new insights into the clinical phenotypes, genetics, clonal evolution, and outcomes in this syndrome, providing invaluable knowledge for understanding its natural history and devising surveillance strategies.
Since the first description of ERCC6L2 syndrome in 2014 in patients with bone marrow failure (BMF),2 20 unique cases have been reported in 6 studies. The affected patients were diagnosed as children or young adults with hypocellular BMF, with approximately half of them presenting with constitutional features, such as microcephaly and developmental delay (see the article by Hakkarainen et al for all previously reported cases). In 2019, Douglas et al described 5 individuals with germline ERCC6L2 mutations who presented with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) with erythroid predominance resembling AML M6.3 For the first time, the authors also established a link between ERCC6L2-related MDS/AML and somatic TP53 mutations. This study expanded the spectrum of this novel predisposition syndrome and revealed that AML can be an initial clinical presentation, leading to the important conclusion: “Our families have acknowledged for years that AML with a dismal prognosis runs among them. This study has finally discovered the culprit and has also supplied some family members with a relieving verdict.”3
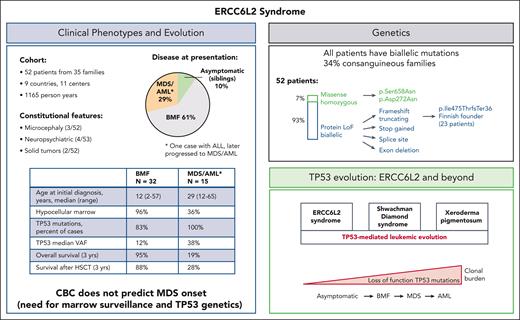
Despite the multiple studies, there was no comprehensive understanding of the clinical spectrum, natural history, and disease progression. The current study by Hakkarainen et al systematically addresses these outstanding questions and successfully integrates the numerous clinical and genetic puzzle pieces. This multicenter retrospective study is exceptional in several ways. First, it includes the largest cohort of patients with this syndrome ever reported (52 cases across 35 families in 9 countries; see figure), which will inform future clinical practice. Second, it provides prognostic estimates for patients at different stages of hematologic disease (BMF, MDS, and AML), aiding in better patient management. Third, it identifies a common Finnish founder mutation and expands the ERCC6L2 mutation spectrum, valuable for genetic counseling and testing. Finally, the study provides insights into surveillance strategies, helpful for early detection and long-term management.
Characteristics of ERCC6L2 disease. Clinical and genetic features of ERCC6L2 disease are shown in the respective boxes. ALL, acute lymphatic leukemia; CBC, complete blood count; HSCT, hematopoietic stem cell transplantation; LoF, loss of function; VAF, variant allelic frequency.
Characteristics of ERCC6L2 disease. Clinical and genetic features of ERCC6L2 disease are shown in the respective boxes. ALL, acute lymphatic leukemia; CBC, complete blood count; HSCT, hematopoietic stem cell transplantation; LoF, loss of function; VAF, variant allelic frequency.
ERCC6L2, also known as RAD26L and helicase mutated in bone marrow failure (HEBO), is a SWI/SNF (SWItch/Sucrose Non-Fermentable)-like ATPase2 and a bona fide nonhomologous end-joining (NHEJ) factor.4 It is a centromeric protein with a role in DNA double-strand break repair via NHEJ pathway, DNA recombination, translocation, and chromatin unwinding.2,5 Cells from patients with biallelic loss-of-function mutations in ERCC6L2 are sensitive to ionizing radiation and phleomycin (both DNA double-strand break-damaging agents) and only weakly sensitive to mitomycin C.6 ERCC6L2 was also demonstrated to participate in RNA polymerase II–mediated transcription to resolve DNA-RNA hybrids (R loops), pointing to ERCC6L2 deficiency as a primary transcription deficiency rather than a stereotypical DNA repair syndrome.7 In the current study, only 3 of 52 patients had microcephaly and developmental delay, which is a common feature of DNA repair disorders with disrupted NHEJ pathway. Likewise, other features common of DNA repair syndromes, such as immunodeficiency or high prevalence of lymphatic and solid tumors, were lacking. In this cohort, hypocellular BMF with cytopenia was the most common initial manifestation in nearly two-thirds of patients, followed by MDS/AML in 29% and asymptomatic state in 10%. The median age in patients presenting with BMF was 12 years, whereas patients with MDS/AML were older, with a median age of 29 years at initial presentation, and 37 years for all patients with MDS/AML, including patients who progressed (Hakkarainen et al, Table 1). Compared with BMF, the MDS/AML cohort was characterized by a higher number of patients carrying somatic loss-of-function TP53 mutations and a higher TP53 variant allelic frequency, pointing to a TP53-mediated clonal progression similar to what has been recently observed in Shwachman-Diamond syndrome8 and xeroderma pigmentosum.9
In patients with inherited BMF syndromes, annual bone marrow surveillance is usually conducted to detect leukemic evolution, with complete blood counts (CBCs) performed in between; changes in CBC might suggest AML progression, prompting an earlier bone marrow examination. However, this strategy is not applicable in ERCC6L2 disease, as CBC abnormalities appear mild despite the presence of TP53-mediated clonal evolution or marrow dysplasia, which is analogous to Shwachman-Diamond syndrome. This study highlights the importance of incorporating bone marrow assessments and high-sensitivity TP53 mutation testing into the clinical follow-up of ERCC6L2-affected individuals.
The present study has implications for genetic diagnostics and counseling. One-third of the affected families were consanguineous, which was defined as parents being second cousins or closer. Most patients (93%) had biallelic (homozygous or compound heterozygous) protein loss-of-function ERCC6L2 mutations in the form of frameshift truncating, stop gained, splice site, and exon deletion, whereas missense homozygous mutations were found in 7% (see figure). Larger cohorts of patients will be needed to investigate the biological effect of different mutations and to determine whether there is a correlation between the genotypes and latency to develop leukemia.
The current work is limited by its retrospective nature, which may have resulted in bias in the outcome estimates. Notably, patients with TP53-mutated MDS/AML had a dismal overall survival rate of 19% and a survival rate after hematopoietic stem cell transplantation (HSCT) of 28%. Prospective studies focusing on earlier diagnosis of MDS/AML and expeditious HSCT are warranted to address this issue and improve outcomes of patients with ERCC6L2 disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal