Abstract
T cells expressing chimeric antigen receptors (CARs) have achieved major clinical success in patients with hematologic malignancies. However, these treatments remain largely ineffective for solid cancers and require significant time and resources to be manufactured in an autologous setting. Developing alternative immune effector cells as cancer immunotherapy agents that can be employed in allogeneic settings is crucial for the advancement of cell therapy. Unlike T cells, Vα24-invariant natural killer T cells (NKTs) are not alloreactive and can therefore be generated from allogeneic donors for rapid infusion into numerous patients without the risk of graft-versus-host disease. Additionally, NKT cells demonstrate inherent advantages over T-cell products, including the ability to traffic to tumor tissues, target tumor-associated macrophages, transactivate NK cells, and cross-prime tumor-specific CD8 T cells. Both unmodified NKTs, which specifically recognize CD1d-bound glycolipid antigens expressed by certain types of tumors, and CAR-redirected NKTs are being developed as the next generation of allogeneic cell therapy products. In this review, we describe studies on the biology of NKTs and other types of innate-like T cells and summarize the clinical experiences of unmodified and CAR-redirected NKTs, including recent interim reports on allogeneic NKTs.
Introduction
Natural killer T (NKT) cells are a subset of innate-like T lymphocytes that recognize glycolipids presented by the monomorphic major histocompatibility complex (MHC)-like molecule CD1d.1,2 Type I NKTs express invariant T-cell receptor (TCR) α-chain TRAV10+TRAJ18+ (formerly Vα24-Jα18), which is preferentially paired with the TRBV25 TCR β chain (formerly Vβ11).3,4 This subset of NKTs can be identified using monoclonal antibody clone 6B11, by reactivity to synthetic glycolipid alpha-galactosylceramide (αGalCer), and using CD1d/αGalCer tetramers.5 Type II NKT cells express a diverse TCR repertoire and recognize both glycolipids and phospholipids presented by CD1d, but antigen specificity for these cells remains poorly defined and does not include αGalCer.6 As this review focuses on type I NKTs, all mentions of NKTs from this point will refer to type I invariant cells.
Although there are many examples in the literature that use T and NK cell markers, including CD3+CD161+ and/or CD3+CD56+ phenotypes to define human NKTs, such phenotypes are not associated with any defined lymphocyte subsets. T cells (both conventional and γ/δ) and NKT cells alike express CD56, CD161, and other NK cell markers, particularly as they undergo terminal effector differentiation.7 In addition, chronically stimulated conventional T cells upregulate NK markers in the context of infection,8 cancer,9 and adoptive transfer for cancer immunotherapy.10 Therefore, NKT cells are defined by CD1d-restricted antigen recognition and not co-expression of T and NK cell markers.
Ontogenetically, NKTs are long-lived innate-like T lymphocytes that develop in the thymus and are positively selected by CD1d-expressing thymocytes.11,12 Expression of the transcription factor PLZF following positive selection drives the NKT effector program and induces the spontaneous acquisition of effector/memory function in these cells.13,14 NKTs participate in various types of immune responses, from protecting against viral and bacterial infection to regulating autoimmunity by sensing both microbial and endogenous glycolipids.15-18 For example, NKTs are required for immunity against gram-negative bacteria that do not contain TLR-4 activating lipopolysaccharide, including Sphingomonas and Borrelia, which instead contain NKT-activating ligands.16,19
NKTs form a crucial bridge between innate and adaptive immunity, producing large amounts of polyfunctional cytokines within hours of activation20 that protect the host from infection and tumor growth. In this review, we describe the antitumor function of NKT cells and their potential use as cancer immunotherapy agents, including recent clinical advances. We also explored studies of other unconventional T-cell subsets that are being developed for use as immunotherapy effectors.
Mechanisms of NKT cell antitumor activity
Several studies have shown significant positive associations between numbers and/or functional activity of tumor-infiltrating/circulating NKTs and clinical outcomes in patients with cancer, including those with neuroblastoma,21 colon cancer,22 multiple myeloma,23 and lung cancer.24 Conversely, tumor progression is often accompanied by a decrease in NKT number, decline in NKT function, and/or downregulation of CD1d expression in malignant cells.25-28 CD1d is expressed by hematopoietic cells, and some hematologic malignancies retain CD1d expression, particularly those of myelomonocytic and B-cell origin.28-31 NKTs have also been shown to kill malignant lymphoid/myeloid cells in vitro. 23,29,32 Some solid tumors, such as high-grade gliomas and medulloblastoma, also express CD1d and can be directly targeted by NKTs.33,34 Regardless of CD1d expression, NKTs effectively localize to tumor sites in response to chemokines such as CCL2 and CCL20, which are produced by many types of tumor cells and/or tumor stromal cells.21,35,36 After infiltrating tumor tissues, NKTs participate in several interactions that indirectly mediate antitumor immune responses, which are crucial for NKT antitumor activity, because most tumors are CD1d-negative. For example, NKTs interact with CD1d+ tumor-associated macrophages (TAMs), either killing them or polarizing protumor M2-like TAMs into antitumor M1-like TAMs.37-39 NKTs also convert myeloid-derived suppressor cells in the tumor microenvironment into immunostimulatory antigen-presenting cells.40 In addition, NKTs stimulate maturation of dendritic cells (DCs) via CD40-CD40L interaction, licensing DCs for effective cross-presentation of tumor-derived antigens to CD8 T cells and ultimately potentiating tumor-specific immunity.41-43 NKT-activated DCs also produce IL-12, leading to increased NK cell-mediated IFNγ production and tumor cell lysis.44
Therapeutic development using endogenously activated or adoptively transferred autologous NKTs
In the clinic, in vivo activation of endogenous NKTs has been evaluated in early-stage clinical trials for several types of cancer. In one such trial, 24 patients with various solid tumors were treated with αGalCer to activate endogenous NKTs; cytokine production (TNFα, GM-CSF) increased in some patients and no dose-limiting toxicities were observed.45 Seven patients in this trial achieved a stable disease status, but no objective responses were recorded. Several other trials instead evaluated DCs or antigen-presenting cells pulsed ex vivo with αGalCer in patients with multiple myeloma or non-small cell lung cancer (NSCLC).46-49 Although some patients showed signs of immune activation, the best clinical outcome achieved in these trials was stable disease.
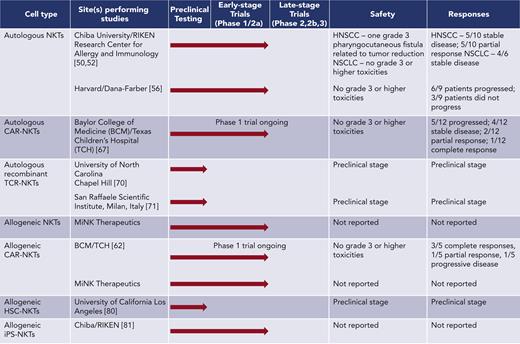
Whereas in vivo activation results in limited expansion of endogenous NKTs in patients, adoptive transfer of exogenously expanded NKTs allows for the infusion of larger numbers of NKTs and permits genetic modification of cells before infusion. Because of the low frequency of NKTs in the peripheral blood, specialized protocols have been developed to generate clinically useful numbers of NKTs ex vivo. In the first method, αGalCer and IL-2, with or without GM-CSF, were added directly to peripheral blood mononuclear cells, resulting in the expansion of NKTs along with other activated lymphocytes. Autologous cell products generated using these methods had a range of 0.59% to 45.45% NKT purity (average 11%) and were tested in early-stage trials in patients with NSCLC48,50 and head and neck squamous cell carcinoma (HNSCC).51,52 The infusions were well tolerated and produced partial responses in some patients with HNSCC (Figure 1).
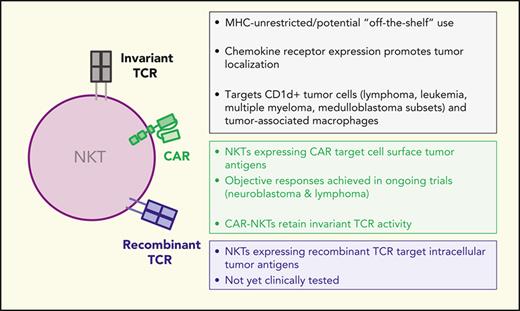
Development of unmodified NKTs and engineered NKTs for cancer immunotherapy. Unmodified and engineered NKTs are being tested in preclinical models and at the indicated clinical trial stages in patients with cancer with safety and clinical responses reported when applicable. BCM, Baylor College of medicine; HSC, hematopoietic stem cell transplants; iPSC, induced pluripotent stem cell.
Development of unmodified NKTs and engineered NKTs for cancer immunotherapy. Unmodified and engineered NKTs are being tested in preclinical models and at the indicated clinical trial stages in patients with cancer with safety and clinical responses reported when applicable. BCM, Baylor College of medicine; HSC, hematopoietic stem cell transplants; iPSC, induced pluripotent stem cell.
NKT cell expansion protocols that have been developed in the last decade often include a step to magnetically sort NKT cells from peripheral blood mononuclear cells, followed by stimulation with αGalCer-pulsed antigen-presenting cells and culture with IL-2 alone or in combination with other cytokines.53-55 In other recent methodologies, αGalCer is replaced with agonistic monoclonal antibodies to stimulate the proliferation of magnetically sorted NKTs, followed by culture with various cytokines.56,57 These protocols generally produce larger, purer populations of NKTs compared with earlier methods, with a range of 13% to 87% NKT purity (average 54%). A recent pilot clinical trial of autologous NKTs that were ex vivo-expanded using OKT3 monoclonal antibody stimulation demonstrated that the infusions were well tolerated but did not produce objective responses in melanoma patients56 (Figure 1). Therefore, although NKT ligands and ex vivo-expanded purified NKTs produce immune responses in patients with cancer without causing notable toxicity, these therapies do not control tumor growth in a clinically significant manner.
Therapeutic development using redirected autologous NKTs
Several strategies have emerged to redirect NKT cell specificity toward cancer cells while preserving their natural antitumor properties, including CD1d-antibody fusion proteins, CARs, and tumor-specific TCRs.
CD1d-antibody fusion proteins combine an αGalCer-loaded CD1d molecule with a tumor antigen-specific single chain variable fragment. These complexes can be used to activate NKTs in vivo in the presence of tumor antigens and to redirect NKTs against specific tumor antigens. For example, CD1d-antibody fusion proteins have been used in preclinical models to target HER2 in melanoma and colon cancer, CEA in colon cancer, and CD19 in lymphoma. Mice treated with these fusion proteins demonstrated elevated NKT cell numbers, increased proinflammatory cytokine production, and showed evidence of tumor regression.58-61
Recent clinical studies have shown that CAR-T cells can mediate complete remission in patients with hematologic malignancies. However, CAR-T cells remain ineffective in solid cancers, constituting a fundamental need for different CAR-based strategies to redirect immune effectors against solid malignancies. NKTs have been engineered to express CARs for several tumor-associated antigens including CD19, CD38, BCMA, CSPG4, and GD2.53,62-64 CAR-NKTs demonstrate similar levels of in vitro cytotoxicity to CAR-T cells and mediate potent antitumor activity in murine models.53,54,63-66 NKTs expressing a GD2-specific CAR were shown to traffic to neuroblastoma (NB) tumors more effectively than GD2–CAR-T cells53; GD2–CAR-NKTs also retained the ability to target M2 macrophages and CD1d-expressing tumor cells via the invariant TCR.53,63,65,66 Further, we found that CAR-NKT cell antitumor activity can be enhanced by coexpressing transgenic IL-15 in a NB tumor mouse model without causing toxicity.65 Based on these preclinical data, we initiated a phase I clinical trial evaluating autologous NKTs co-expressing a GD2-specific CAR (GD2-CAR) and IL-15 in patients with relapsed/refractory NB (NCT03294954). Emerging results from this trial show that GD2–CAR-NKTs are safe, expand in the peripheral blood after infusion, traffic to tumor sites, and mediate objective responses in patients with NB (Figure 1).67,68
NKTs can also be engineered to express antigen-specific TCRs for use in adoptive cell therapies. Initial studies expressed a Mycobacterium-specific TCR in NKTs and showed that these cells recognized DCs pulsed with either Mycobacterium antigens or αGalCer in vitro.69 More recently, NKTs have been engineered to express TCRs specific to various tumor antigens. One study revealed that TCR avidity is crucial and that NKTs expressing low-avidity TCRs (PRAME- and MART1-TCRs) had less potent cytolytic functions than T cells expressing the same TCRs; NKTs expressing the high-avidity Tyr-TCR, instead, demonstrated potent antitumor activity in a murine model.70 Another recently published study demonstrated that TCR-engineered NKT cells (TCR-NKTs) were more effective than non-transduced NKTs and CD8+ T cells expressing the same TCR in controlling the progression of multiple types of tumors expressing the cognate antigen. Mechanistically, TCR-NKTs mediated robust cancer control in that study by simultaneously modulating intratumoral suppressive myeloid populations and killing malignant cells.71 Clinical trials studying TCR-modified NKTs in patients with cancer have not been initiated to date.
Therapeutic development using allogeneic NKTs
Because NKTs are restricted by monomorphic CD1d and not HLA molecules, they are inherently nonalloreactive. Therefore, therapeutic NKTs can be generated from allogeneic donors and used in different individuals without the risk of graft-versus-host disease (GVHD). In fact, the presence of NKTs in allogeneic hematopoietic stem cell transplants has been shown to protect against GVHD while maintaining graft-versus-tumor activity. Studies evaluating NKT frequency in graft cell content showed that lower NKT frequency, particularly CD4− NKTs, correlated with the incidence of grade II-IV GVHD.72,73 In one study, NKT frequency in graft products correlated with GVHD-free and progression-free survival.74 Clinical trials have shown that implementing methods to preserve the NKT compartment in hematopoietic stem cell transplant graft products, including conditioning regimens that preserve NKT recovery, decreases the incidence of GVHD.75,76 These studies suggest that NKTs are uniquely suited for use in allogeneic adoptive cell therapy as effector cells that do not induce GVHD and may even reduce the risk of GVHD from contaminating T cells in infusion products.
Several groups have evaluated unmodified allogeneic NKTs as immunotherapies for CD1d+ malignancies, including multiple myeloma. A phase I clinical trial for relapsed/refractory multiple myeloma that treats patients with unmodified NKTs from an allogeneic bank is currently ongoing (NCT04754100). Another phase I trial from this group used the same allogeneic NKT bank to treat patients with COVID-ARDS (NCT04582201) and observed no dose-limiting toxicities, neurotoxicity, or cytokine release syndrome up to a dose of 1 × 109 cells, suggesting that allogeneic NKTs are safe even at high doses.77 Several studies of modified NKTs from allogeneic donors are underway as well. Murine CD19-specific CAR-NKTs demonstrated both direct cytotoxic activity as well as the ability to cross-prime tumor-specific CD8+ T cells when infused into MHC-mismatched mice.78 In an ongoing clinical trial at our center for relapsed/refractory CD19+ malignancies (NCT00840853), allogeneic CD19-CAR-NKTs have thus far been well tolerated and have mediated a high rate of objective responses even at low dose levels (Figure 1).62
Although large-scale clinical expansion of peripheral blood NKTs is feasible,56,62,66,67,77 recent efforts have been made using stem cell techniques to engineer large banks of NKT-like cells. One such strategy uses engineered hematopoietic stem cells to express invariant NKT-TCR. Following transfer into humanized BLT mice, these NKT-TCR+ HSCs can give rise to NKT-TCR+ T cells that are phenotypically similar to NKTs; these cells express the NKT master regulator PLZF and produce both Th1 and Th2 cytokines as well as cytotoxic molecules.79 Recently, Li et al cultured NKT-TCR+ HSCs in an in vitro artificial thymic organoid system and then stimulated the cells with αGalCer to expand a bank of allogeneic HSC-NKTs for adoptive cell therapy.80 The resulting cells phenotypically resembled endogenous NKTs because of the expression of memory, NK cells, and tissue-homing markers. Notably, HSC-NKTs did not contain the CD4+ subset observed in endogenous NKTs, and showed a marked increase in NK-activating receptors. Ultimately, this strategy yielded a large bank of allogeneic HSC-NKTs, with up to 1011 cells from cord blood-isolated HSCs and 1013 cells from peripheral blood stem cells. These cells demonstrated antitumor activity in a human melanoma xenograft model. Another strategy for producing large banks of NKTs involves the use of NKT cell-derived iPSCs. NKTs can then be re-differentiated from NKT-iPSCs, and the resulting NKTs retain the ability to produce IFNγ, exhibit robust in vitro cytotoxic activity, have similar antitumor activity in a human xenograft mouse model compared to parental NKTs.81 iPSC-derived NKTs are currently being tested in a clinical trial for patients with recurrent or advanced head and neck cancer (jRCT2033200116).
Although NKTs are not alloreactive, as with any type of allogeneic cell, they are still subject to rejection by the infused patient due to the expression of mismatched HLA molecules. One strategy to overcome this limitation involves knocking out or knocking down expression of HLA class I and II molecules in NKTs to avoid T cell–mediated rejection. However, eliminating HLA class I expression increases the risk of NK cell-mediated rejection and knockdown strategies are more likely to address both T- and NK-mediated rejection. Indeed, allogeneic HSC-NKTs, which have reduced HLA class I expression and no class II expression, have reduced T- and NK-mediated rejection in in vitro mixed lymphocyte reaction assays.80 In the clinic, HLA knockdown has been employed to prevent rejection of therapeutic NKTs in an ongoing allogeneic CAR-NKT clinical trial that uses shRNAs targeting B2M and CD74 to reduce expression of class I and II molecules, respectively.62 Other strategies to limit rejection of allogeneic cells include expression of the “don’t-eat-me” signal CD47,82 expression of the NK cell-inhibitory molecule HLA-E,83 and production of multiple allogeneic banks to promote partial HLA matching84; these strategies have not yet been evaluated in NKTs.
Therapeutic development using other innate-like T lymphocytes
In addition to NKTs, other unconventional T-cell subsets may also have advantages for use as allogeneic cancer immunotherapeutic agents. These cells, sometimes referred to as donor unrestricted T cells (DURTs),85 include γδ-T cells and MR1-restricted cells, such as mucosal-associated invariant T (MAIT) and MR1-T cells, in addition to CD1-restricted T cells. Like NKTs, these cells typically express the master regulator PLZF, are not restricted by MHC, recognize non-peptidic antigens, and are present at higher frequencies in tissues than in peripheral blood. The extent to which these cell types are characterized in terms of defined ligands and antitumor potential varies. For example, γδ-T cells, particularly the Vδ2 subset, and NKTs have defined ligands and well-characterized ex vivo expansion protocols, whereas MAIT cells have defined ligands, but lack an optimized ex vivo expansion protocol.
Studies on MR1-restricted T cells, including both MAIT and MR1-T cells, have been conducted over the past decade. Similar to CD1d-restricted cells, MR1-restricted T cells do not induce GVHD and therefore could potentially be used as off-the-shelf products. MAIT cells express a semi-invariant TCR that recognizes vitamin B-related ligands present in the context of MR1.86 These cells localizes to mucosal tissue but are also present in the peripheral blood, liver, and lymphoid tissues. MAIT cells participate in protecting against bacterial and viral infection,87,88 but their role in cancer is less clear and may depend on the tumor type. MAITs have been reported to accumulate at tumor sites in patients with colorectal cancer,89-91 but greater MAIT accumulation was shown to correlate with worse disease prognosis90; in patients with hepatocellular carcinoma, lower MAIT numbers at the tumor site instead correlated with worse prognosis.92 For hematologic malignancies, in vitro evidence suggests that MAIT cells possess antitumor activity with an ability to target multiple myeloma cells.93 Although MAIT cells remain underdeveloped for use in the clinic, several lines of evidence suggest that MAITs or CAR-MAITs could be effective cancer immunotherapy agents. A preliminary study expressing a mesothelin-targeting CAR in MAIT cells showed that these cells mediate specific antitumor activity in vitro, but further in vitro characterization and in vivo testing are needed to elucidate the clinical potential of CAR-MAITs.94
Unlike MAIT cells, MR1-T cells express a diverse TCR repertoire and are present at a much lower frequency in the peripheral blood. These cells do not react to microbial antigens or vitamin B metabolites, but multiple clones have been identified that respond to tumor-associated antigens.95 One of which can target multiple human cancer cell lines in vitro in an MR1-dependent manner (including lung, melanoma, leukemia, colon, breast, prostate, bone, and ovarian cell lines), but does not affect healthy human tissue. T cells transduced with this specific MR1-T TCR also had a protective effect in a xenograft leukemia mouse model,96 suggesting that this TCR could be promising for off-the-shelf cancer therapy. Some evidence suggests that MR1-T cells recognize antigens associated with metabolic stress, although specific antigens have not been identified, and this theory remains to be tested.97 MR1-T cells are in the early stages of characterization but are promising for use in cancer immunotherapy because of their natural antitumor properties.
Another example of unconventional T cells that lack MHC-restriction are γδ-T cells, which can be divided into 3 subsets based on delta chain rearrangements: Vδ1, Vδ2, and Vδ3. Vδ1 and Vδ3 γδ-T cells are found in the intestinal mucosa, skin, and liver98,99 whereas Vδ2 γδ-T cells have a higher frequency in the peripheral blood.100 The mode of activation for γδ-T cells remains poorly studied, and similarly, TCR restriction and activating antigens are still being defined. Vδ1 and Vδ3 TCRs bind to CD1 and MR1,101 and Vδ2 cells recognize phosphoantigens102,103 presented by a member of the butyrophillin protein family.101,104 In vitro, γδ-T cells are cytotoxic against a variety of cancer cell types, often by engaging stress-induced molecules, MICA/MICB, which are upregulated in transformed cells.103,105 In the context of human cancers, infiltrating γδ-T cells have shown both pro and antitumorigenic properties. For example, IL17-producing γδ-T cells correlated with decreased survival in 2 studies,106,107 whereas a meta-analysis across 39 malignancies showed that intra-tumoral γδ-T cells had the most significant positive correlation with survival of all evaluated immune cell populations.108
The clinical development of autologous γδ-T cells for use in adoptive therapy is becoming more common, as these cells can be expanded ex vivo using synthetic phosphoantigens or bisphosphonates, such as zoledronic acid. For example, ex vivo-expanded Vδ2 γδ-T cells infused into patients with renal carcinoma,109,110 gastric cancer,111 colorectal cancer,112 and NSCLC113,114 were safe and well tolerated, but objective responses were minimal. Historically, the Vδ1 subset has been less studied in the clinic because of its lower peripheral blood numbers and lack of defined antigens. However, these cells can be expanded in vitro by first sorting for Vδ1 cells and then stimulating with either the CD3 agonist OKT3115 or concanavalin-A.116 An agonistic anti-Vδ1 antibody has also recently been developed to facilitate the expansion of Vδ1 T cells, enabling ongoing clinical studies of expanded Vδ1 T cells, including those expressing CARs.117,118 With the clinical development of γδ-T cells for adoptive cell therapy, there are plans to use these cells as a platform for CAR expression116,117 in autologous and allogeneic settings. An ongoing phase I clinical trial for patients with relapsed/refractory B-cell lymphoma (NCT04735471) has shown that allogeneic γδ-T cells expressing a CD20-specific CAR do not induce GVHD and have comparable safety to autologous CAR-T cells; 4 of 6 patients in this trial achieved complete responses.118 Following these promising results, additional trials evaluating allogeneic γδ-T cells are ongoing or planned for NB (NCT05400603), hepatocellular carcinoma (NCT04518774), and other solid tumors (NCT04107142).
Summary
In conclusion, NKTs participate in the antitumor immune response through direct CD1d-dependent tumor cytotoxicity or indirectly via interactions with TAMs, DCs, and activation of T and NK cells. However, the antitumor activity mediated by endogenous NKTs or adoptively transferred unmodified NKTs has been limited and may be enhanced by approaches to redirect NKTs using synthetic tumor-specific receptors.
Redirected NKT therapy enables direct tumor cell killing via an engineered receptor, such as CAR or TCR, while simultaneously harnessing the intrinsic antitumor properties and tumor-homing abilities of NKTs. Therapeutic CAR-NKTs can be produced using current manufacturing practices for use in patients in autologous and allogeneic settings, and emerging interim results from ongoing early-stage clinical trials demonstrate that CAR-NKTs can produce objective responses in patients with cancer without causing significant toxicity (Figure 1). NKTs derived from the peripheral blood of healthy donors, cord blood, HSCs, or iPSCs are attractive candidates for the development of next-generation off-the-shelf cancer immunotherapy because of their inherent anti-GVHD properties and lack of alloreactivity. Moreover, the successful development of NKT-based therapies may serve as a template for exploiting the unique properties of other innate-like T cells and the broader spectrum of innate lymphocytes/leukocytes in future cell therapies against cancer and other diseases.
Acknowledgments
The authors acknowledge Erica J. Di Pierro for assistance in editing the article.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (RO1 CA262250 and P50 CA126752) and by Athenex, Inc.
Authorship
Contribution: A.N.C., G.T., and L.S.M. contributed to the conception, design, and composition of the article and interpretation of the literature.
Conflict-of-interest disclosure: A.N.C., G.T., and L.S.M. are coinventors on pending patent applications that relate to the use of NKTs in cancer immunotherapy, including those licensed by Baylor College of Medicine to Athenex, Inc for commercial development. Athenex, Inc provided research support to L.S.M. via a sponsored research agreement with the Baylor College of Medicine.
Correspondence: Leonid S. Metelitsa, Department of Pediatrics, Baylor College of Medicine, 1102 Bates Ave, C.1760.06, Houston, TX 77030; e-mail: lsmeteli@txch.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal