In this issue of Blood, Bennett et al1 explore the potential of dual targeting of interleukin-1 receptor-associated kinase 1 and 4 (IRAK1/4) in hematologic malignancies, such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). This study not only gives important mechanistic insights into how immune signaling pathways are hijacked during MDS/AML development but also offers a new therapeutic approach.
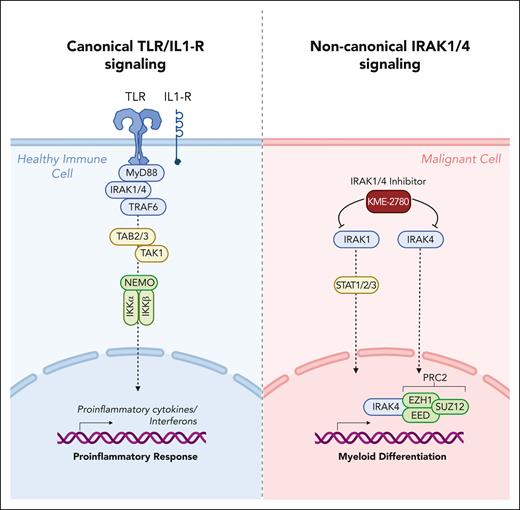
Myeloid malignancies, including MDS and AML, continue to present significant challenges for patients and clinicians due to their complex genetic and molecular landscapes. Recent advances in targeted therapies have shown promise, but durable responses remain elusive for many patients.2 MDS and AML exhibit extensive dysregulation of immune and inflammatory pathways and have many genetic alterations affecting Toll-like receptor (TLR) and interleukin 1 receptor (IL-1R) pathways.3 These pathways converge at IRAK1/4, essential components of the innate immune response (see figure). As it was believed that a complex with MyD88 and IRAK4 is vital for activation of IRAK1 and subsequent downstream effectors during malignant hematopoiesis, IRAK4 inhibitors—such as CA4948 (emavusertib)—and proteolysis targeting chimeric small molecule degraders are being assessed in preclinical studies and clinical trials for hematologic malignancies, including lymphoma, MDS, and AML.4-6
Schematic overview of canonical TLR/IL1-R signaling pathway in healthy cells vs alternative signaling routes mediated by IRAK1/4 in malignant hematopoietic cells described by Bennett et al. Figure created with BioRender.com.
Schematic overview of canonical TLR/IL1-R signaling pathway in healthy cells vs alternative signaling routes mediated by IRAK1/4 in malignant hematopoietic cells described by Bennett et al. Figure created with BioRender.com.
Bennett and colleagues now show that the promising but limited responses to IRAK4 inhibitors in MDS and AML clinical trials are due to functional complementation and compensation by its paralog, IRAK1.7 Their findings indicate that cotargeting IRAK1 and IRAK4 is required to fully suppress leukemic stem/progenitor cell (LSPC) function and induce differentiation (see figure). This is a significant discovery, as it implies that dual IRAK1/4 inhibition provides a more effective therapeutic approach for hematologic malignancies than targeting either molecule alone. Furthermore, the study challenges conventional understanding of IRAK1 and IRAK4 signaling, which was thought to function primarily downstream of the proximal adapter MyD88. The authors discovered that complementary and compensatory IRAK1 and IRAK4 dependencies in MDS/AML occur via noncanonical MyD88-independent pathways, underlining the complexity of immune signaling in hematologic malignancies. Through extensive genomic and proteomic analyses, the authors unveiled that IRAK1 and IRAK4 preserve the undifferentiated state of MDS/AML LSPCs by coordinating a network of pathways, including those converging on the PRC2 complex and JAK-STAT signaling. These findings provide valuable insights into the molecular mechanisms underpinning dysregulated immune signaling in hematologic malignancies. More importantly, these findings provide a strong rationale for developing dual IRAK1/4 inhibitors. Therefore, the authors engineered a potent and selective dual IRAK1 and IRAK4 inhibitor, KME-2780. In preclinical studies, they demonstrated that MDS/AML cell lines and patient-derived samples showed significant suppression of LSPCs in vitro and in xenograft studies in vivo when treated with KME-2780, as compared with selective IRAK4 inhibitors. Thus, this IRAK1/4 inhibitor, KME-2780, warrants further investigation in clinical studies.
The article by Bennett et al has far-reaching implications for leukemia research and treatment. The authors have identified a novel mechanism of resistance to IRAK4 inhibitors, which is hijacking components of canonical immune signaling pathways and rerouting them to block differentiation of hematopoietic cells. This study highlights the significance of considering functional complementation by paralogs and compensatory signaling pathways in the development of targeted therapies. Given that approximately 70% of human protein-coding genes have at least 1 paralog, exploring compensatory mechanisms involving paralogs or noncanonical routes of signaling cascades should be a crucial component in the early stages of drug development.8,9 In some cases, a dual treatment strategy targeting multiple paralogs of a signaling molecule may prove more effective than specific single-target molecules, thereby circumventing potential resistance mechanisms and enhancing patient outcomes. A similar phenomenon was recently demonstrated for MDM2 and its paralog MDMX, both of which can inactivate TP53; dual targeting of MDM2/MDMX exhibited superior therapeutic efficacy in AML.10
In conclusion, Bennett et al’s study represents a significant advancement in our understanding of immune signaling dysregulation in hematologic malignancies and may influence the development of targeted therapies beyond MDS and AML.
Conflict-of-interest disclosure: J.-H.K. has advisory roles for bluebird bio, Boehringer Ingelheim, Novartis, Roche, and Jazz Pharmaceuticals. H.J.U. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal