In this issue of Blood, Carter et al1 report that targeting the epichaperome boosts the effectiveness of venetoclax in treating acute myeloid leukemia (AML), and it also inhibits the outgrowth of venetoclax-resistant TP53-mutant AML.
Venetoclax, an inhibitor of anti-apoptotic protein B-cell lymphoma-2 (BCL-2), has emerged as a promising treatment for AML and is currently considered the standard of care when combined with azacitidine for newly diagnosed patients with AML who are not eligible for induction chemotherapy.2 However, the issue of resistance poses a significant impediment in care of patients undergoing venetoclax treatment, persistently frustrating patients, clinicians, and researchers. The development of resistance is frequently linked to mutations in TP53, RAS, or FLT3 pathways.3 Addition of venetoclax to azacitidine does not confer any significant advantages in TP53-mutated AML cases with poor-risk cytogenetics.4 The article by Carter et al focuses on this critical area, where there is a substantial gap in meeting the medical needs of patients with TP53-mutant AML. After conducting a high-throughput drug screen, they suggest targeting epichaperomes whose inhibition would prevent the outgrowth of venetoclax-resistant cells.
The epichaperome is a sophisticated interconnected network consisting of chaperones and cochaperones found within various tumors. This network promotes the survival of malignant cells.5 Heat shock protein 90 (HSP90) and heat shock cognate protein 70 act as key nucleating sites for these physically and functionally interconnected complexes.5 PU-H71 is a novel epichaperome inhibitor that selectively targets HSP90 in cancer cells expressing the epichaperome.
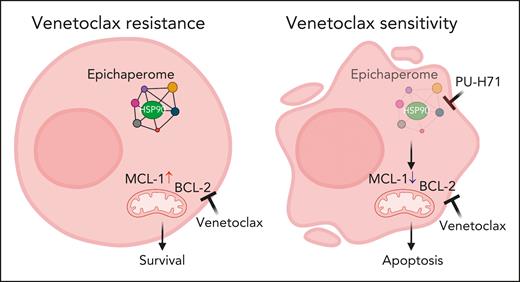
Through an extensive series of experiments, using human leukemia cell lines, primary AML cells in vitro and in xenografts, and patient-derived xenograft (PDX) models, the authors demonstrate the presence of epichaperomes in TP53-mutant AML and AML stem/progenitor cells. Inhibition of the epichaperome using PU-H71 affects TP53-mutant AML and leads to cell death in AML cells and TP53-mutant stem/progenitor cells. Notably, normal bone marrow cells do not exhibit the epichaperome and are, thus, unaffected by its inhibition. On the basis of these data, Carter et al evaluated combination therapy of PU-H71 and venetoclax. They found these 2 agents synergistically induce cell death in AML cells and stem/progenitor cells in both TP53 wild-type and TP53-mutant cells. The study also reveals that PU-H71 decreases myeloid cell leukemia-1 (MCL-1) and increases Bcl-2-interacting mediator of cell death (BIM), thereby enhancing venetoclax activity (see figure). In addition, several other signaling proteins, such as phosphorylated STAT3, phosphorylated protein kinase B, phosphorylated extracellular signal-regulated protein kinase, v-Myc avian myelocytomatosis viral oncogen homolog (c-MYC), heat shock transcription factor-1 (HSF-1), and hypoxia inducible factor-1a (HIF-1a), are downregulated with PU-H71 treatment.
Targeting the HSP90 epichaperome synergized with BCL-2 inhibition to effectively eliminate TP53-mutant AML cells. (Left) Exposure to venetoclax over longer time, MCL-1 expression is elevated. (Right) Addition of PU-H71 decreases MCL-1 expression and induces apoptosis. Figure created with BioRender.com.
Targeting the HSP90 epichaperome synergized with BCL-2 inhibition to effectively eliminate TP53-mutant AML cells. (Left) Exposure to venetoclax over longer time, MCL-1 expression is elevated. (Right) Addition of PU-H71 decreases MCL-1 expression and induces apoptosis. Figure created with BioRender.com.
Another study that examined samples from patients with de novo and relapsed/refractory primary AML also reported a high level of epichaperomes in nearly 50% of the samples.6 Furthermore, there was a recent report of a patient with a novel PML-SYK fusion AML who achieved durable complete remission after PU-H71 treatment.7 Phase 1 clinical trials evaluating PU-H71 in metastatic breast cancer, lymphoma, and myeloproliferative neoplasms have been completed in recent years (clinicaltrials.gov identifiers NCT03166085 and NCT01393509). The findings by Carter et al suggest that AML with mutant TP53 should also be added to the list of target malignant cancers for exploration with PU-H71 given alone or in combination with venetoclax. However, further work is needed to optimize the dosage for combination therapy, as PU-H71/venetoclax did not extend the survival of nonobese diabetic severe combined immunodeficiency gamma SGM3 (NSGS) mice in the PDX model, despite effectively reducing disease burden. In addition, combination therapy needs to be explored in the laboratory in the 17% of patient samples with AML that are resistant to PU-H71.6 Last, evaluating the abundance of the epichaperome in each patient before treatment would be recommended for effective personalized treatment.
Overall, the exciting study by Carter et al presents compelling evidence that combining the epichaperome inhibitor PU-H71 with the BCL-2 inhibitor venetoclax is a potent and synergistic drug combination. This discovery opens up new avenues for therapeutic intervention in AML, particularly for patients with TP53 mutations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal