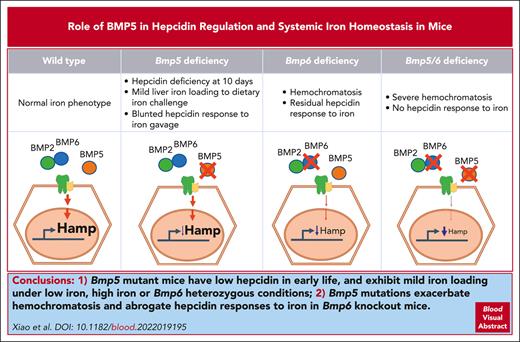
Key Points
Bmp5-mutant mice have low hepcidin in early life and exhibit mild iron loading under low- or high-iron or Bmp6-heterozygous conditions.
Bmp5 mutations exacerbate hemochromatosis and abrogate hepcidin responses to iron in Bmp6-knockout mice.
Abstract
Hepcidin is the master regulator of systemic iron homeostasis. The bone morphogenetic protein (BMP) signaling pathway is a critical regulator of hepcidin expression in response to iron and erythropoietic drive. Although endothelial-derived BMP6 and BMP2 ligands have key functional roles as endogenous hepcidin regulators, both iron and erythropoietic drives still regulate hepcidin in mice lacking either or both ligands. Here, we used mice with an inactivating Bmp5 mutation (Bmp5se), either alone or together with a global or endothelial Bmp6 knockout, to investigate the functional role of BMP5 in hepcidin and systemic iron homeostasis regulation. We showed that Bmp5se-mutant mice exhibit hepcidin deficiency at age 10 days, blunted hepcidin induction in response to oral iron gavage, and mild liver iron loading when fed on a low- or high-iron diet. Loss of 1 or 2 functional Bmp5 alleles also leads to increased iron loading in Bmp6-heterozygous mice and more profound hemochromatosis in global or endothelial Bmp6-knockout mice. Moreover, double Bmp5- and Bmp6-mutant mice fail to induce hepcidin in response to long-term dietary iron loading. Finally, erythroferrone binds directly to BMP5 and inhibits BMP5 induction of hepcidin in vitro. Although erythropoietin suppresses hepcidin in Bmp5se-mutant mice, it fails to suppress hepcidin in double Bmp5- and Bmp6-mutant males. Together, these data demonstrate that BMP5 plays a functional role in hepcidin and iron homeostasis regulation, particularly under conditions in which BMP6 is limited.
Introduction
Systemic iron homeostasis is governed by the liver iron hormone hepcidin.1 Hepcidin binds to the iron exporter ferroportin to induce degradation of the complex, therefore controlling iron release into circulation from dietary sources and body stores.1,2 Hepcidin is downregulated by iron deficiency and erythropoietic drive, and it is upregulated by iron loading and inflammation.3-5 These regulatory systems provide adequate iron supply for erythropoiesis while preventing the toxic consequences of iron overload.3-5 Hepcidin deficiency or excess contributes to most systemic iron homeostasis disorders, including hereditary hemochromatosis, iron-loading anemias, anemia of inflammation, and iron-refractory iron deficiency anemia.6,7
The bone morphogenic protein (BMP)-SMAD signaling pathway is the central mechanism for hepcidin regulation by iron.8 BMP6 and BMP2 ligands are key, known endogenous hepcidin regulators.9-12 Produced and secreted by liver endothelial cells in response to iron loading, BMP6 and BMP2 bind to the coreceptor hemojuvelin (HJV) and BMP receptor complex on hepatocytes to activate SMAD1/5/8 signaling, which directly upregulates hepcidin transcription.9,11-17 Mice with a global or endothelial Bmp6 knockout (KO) or endothelial Bmp2 KO develop hemochromatosis, characterized by hepcidin deficiency and serum and tissue iron overload.9-12,16 Humans with heterozygous mutations in the BMP6 prodomain also develop inappropriate hepcidin expression and iron overload.18,19
BMP6 and BMP2 function together in hepcidin and iron homeostasis regulation because the hemochromatosis phenotype does not worsen in double endothelial Bmp2- and Bmp6-KO mice compared with single endothelial Bmp2- or Bmp6-KO mice.20 Moreover, although a pan-neutralizing BMP antibody completely inhibits hepcidin induction by iron in Bmp6-KO mice,12 iron maintains a similarly strong ability to induce liver SMAD1/5/8 phosphorylation and hepcidin expression in double endothelial Bmp2-KO and global Bmp6-KO mice compared with single Bmp6-KO mice.20 These data suggest that at least 1 additional BMP ligand contributes to hepcidin regulation by iron.
The BMP-SMAD pathway also mediates hepcidin suppression by erythropoietic drive.8 Erythropoietic signals induce erythroid precursors to secrete erythroferrone (ERFE),21,22 which binds directly to BMP ligands to prevent their interaction with the BMP receptor complex, thereby inhibiting hepcidin expression.23 Notably, Epogen (EPO) injection still suppresses hepcidin in Bmp6-KO mice,24 suggesting that there are additional endogenous BMP ligand(s) targeted by ERFE to suppress hepcidin in response to erythropoietic signals. ERFE preferentially inhibits class II BMP ligand homodimers (BMP5, BMP6, and BMP7) or class I/class II heterodimers (including BMP2/6) compared with class I BMP homodimers (BMP2 or BMP4).23,25 BMP5 and BMP7 also bind to HJV with a high affinity13 and stimulate hepcidin expression when exogenously added to hepatocytes,26 although endogenous BMP7 expression in the adult liver is negligible.27
Here, we investigated whether BMP5 contributes to hepcidin regulation and systemic iron homeostasis in vivo by examining the iron phenotype of mice with an inactivating Bmp5 mutation (Bmp5se),28,29 either alone or together with a global or endothelial Bmp6 KO. Mice were also examined after long-term dietary iron manipulation, short-term oral iron gavage, or EPO injection.
Methods
Animals
Bmp5 short-ear (Bmp5se) mice on a C57BL/6J background (The Jackson Laboratory, #000056) were bred with wild-type C57BL/6J mice to generate heterozygotes, which were then intercrossed to generate littermate Bmp5se homozygous mutant mice, Bmp5het heterozygous mice, and Bmp5wt controls. Global Bmp6 heterozygous mice (Bmp6het)16 backcrossed to a C57BL/6 background were bred with Bmp5het mice to generate littermate mice with 0 to 2 Bmp6 alleles and 0 to 2 functional Bmp5 alleles. Endothelial Bmp6 conditional KO (Bmp6fl-Tek-Cre+) mice16 were bred with Bmp5het mice to generate Bmp5het-Bmp6fl-Tek-Cre+ males and Bmp5het-Bmp6fl-Tek-Cre– females, which were crossed to generate littermate mice with endothelial Bmp6 KO and 0 to 2 functional Bmp5 alleles compared with littermate Cre– controls.
Mice were weaned to a Prolab 5P75 Isopro RMH 3000 house diet (380 parts per million [ppm] iron), unless otherwise indicated. Where indicated, 6- to 8-week-old mice received an intraperitoneal injection of 100 μL of phosphate buffered saline or EPO (200 units; Amgen) and were euthanized after 15 hours. Seven-week-old mice were fed on an adequate-iron (48 ppm; Harland #TD.80394) or low-iron diet (2-6 ppm; Harlan #TD.80396) for 10 days. Low-iron diet–fed mice received a single dose of water or 2 mg/kg elemental iron as ferrous sulfate (Sigma 450278) in 0.5 M ascorbic acid via oral gavage for 5 hours. Four-week-old mice were fed on a high-iron diet (1% carbonyl iron; Harlan #TD.08043) for 3 weeks. Littermate Bmp5se-Bmp6fl-Tek-Cre+ and Bmp5se-Bmp6fl-Tek-Cre– mice were weaned to the low-iron diet for 4 weeks or the low-iron diet for 3 weeks, followed by the adequate-iron diet for 1 week. Animal protocols were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
RNA isolation, reverse transcription, and quantitative polymerase chain reaction
Total RNA was isolated, first-strand complementary DNA was synthesized, and quantitative polymerase chain reaction was performed as previously described.30 The relative quantities were determined using the standard curve method.30 For copy number assays, pCAG-Bmp5 and pCAG-Bmp6 (Addgene #163589 and #163590) were used to generate standard curves using the following equation: copy number = plasmid amount × (6.02 × 1023 ÷ plasmid length) ÷ 660, as previously described.31 The primers used are listed in supplemental Table 1.
Complete blood count and iron analysis
Serum iron and unsaturated iron binding capacity were measured using colorimetric assays (Pointe Scientific) to calculate transferrin saturation, per the manufacturer’s instructions. Complete blood count (CBC) and tissue nonheme iron concentrations (per gram of wet weight) were determined, as previously described.30,32
Immunoprecipitation and immunoblot
Immunoprecipitation assays for BMP5 or BMP6 (1-2 μg; R&D Systems, 6176-BM/CF and 507-BP/CF) with Flag-ERFE concentrate (52.9 ng/μL) were performed, and samples were immunoblotted as described,23 using BMP5 (1:1000; R&D Systems MAB6176), BMP6 (1:1000; R&D Systems AF6325), and Flag (1:4000; Sigma F7425) antibodies. Images were captured using the Syngene G:Box mini-imaging system.
Hepcidin induction assay
Hep3B cells were transfected with 200 ng empty vector or Flag-ERFE23 for 48 hours, followed by treatment with 5 ng/μL BMP5 (R&D Systems, 615-BMC-020) in minimal essential media with 1% fetal bovine serum for 6 hours.
Statistics
Means were compared using Student unpaired t test or 1-way or 2-way analysis of variance with Tukey post hoc test using Prism 9 (GraphPad). Data with unequal variances were log-transformed before the analysis. P < .05 was considered significant.
Results
BMP5 contributes to hepcidin regulation in 10-day-old mice
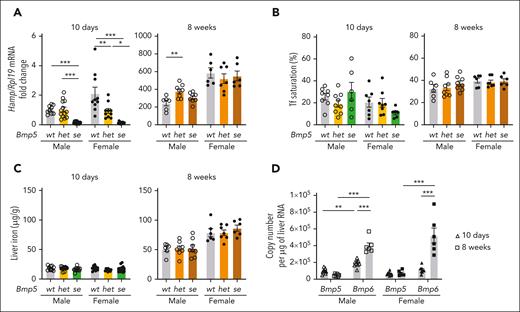
To investigate the role of BMP5 in hepcidin regulation and iron homeostasis, we compared the iron phenotypes of littermate homozygous Bmp5se-mutant mice, heterozygous Bmp5het mice, and wildtype Bmp5wt mice at 10 days and 8 weeks of age. Bmp5se mice have a missense mutation, creating a premature stop codon before the mature BMP5 ligand domain that is required for signaling, resulting in reduced external ear size and other mild skeletal abnormalities.28,29 As validation of the model, whole liver Bmp5 messenger RNA (mRNA) was decreased by ∼50% in Bmp5het mice and 90% in Bmp5se mice compared with that in sex- and age-matched Bmp5wt controls (supplemental Figure 1A, available on the Blood website). No significant differences were observed in liver Bmp6 or Bmp2 mRNA, the known hepcidin regulators, except for slightly reduced Bmp2 in 10-day-old Bmp5se females (supplemental Figure 1B-C).
At 10 days of age, liver hepcidin (Hamp) mRNA expression was significantly lower in both Bmp5se males and females than in sex-matched Bmp5wt controls (∼90%; Figure 1A, left). Bmp5het females had intermediate hepcidin levels, whereas Bmp5het males had hepcidin levels similar to those of the Bmp5wt controls (Figure 1A, left). Bmp5se males had mild increases in erythrocyte mean cell volume and mean cell hemoglobin compared with Bmp5wt controls (supplemental Table 2). However, no other significant differences in CBC parameters, serum iron, serum transferrin saturation, or liver iron levels were observed between sex-matched Bmp5se and Bmp5wt mice (supplemental Table 2; supplemental Figure 1D, left; Figure 1B-C).
BMP5 contributes to hepcidin regulation in 10-day-old mice. (A-C) Ten-day-old and 8-week-old male (open circles) and female (closed circles) Bmp5wt, Bmp5het, and Bmp5se mice were analyzed for liver Hamp relative to Rpl19 mRNA via quantitative polymerase chain reaction (qRT-PCR) (A). The average of the 10-day-old wild-type male mice was set to 1. Serum transferrin (Tf) saturation (B) and liver iron levels (C) were measured using colorimetric assays. (D) Copy number of liver Bmp5 and Bmp6 mRNA was quantified via qRT-PCR in wild-type male (open symbols) and female (closed symbols) mice at ages 10 days (triangles) and 8 weeks (squares). For all graphs, individual data points are shown, and bars represent the mean ± standard error of the mean (SEM). For panels A-C, age- and sex-matched data were analyzed using 1-way analysis of variance (ANOVA) with Tukey post hoc test. For panel D, sex-matched data were analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001 for the indicated comparisons.
BMP5 contributes to hepcidin regulation in 10-day-old mice. (A-C) Ten-day-old and 8-week-old male (open circles) and female (closed circles) Bmp5wt, Bmp5het, and Bmp5se mice were analyzed for liver Hamp relative to Rpl19 mRNA via quantitative polymerase chain reaction (qRT-PCR) (A). The average of the 10-day-old wild-type male mice was set to 1. Serum transferrin (Tf) saturation (B) and liver iron levels (C) were measured using colorimetric assays. (D) Copy number of liver Bmp5 and Bmp6 mRNA was quantified via qRT-PCR in wild-type male (open symbols) and female (closed symbols) mice at ages 10 days (triangles) and 8 weeks (squares). For all graphs, individual data points are shown, and bars represent the mean ± standard error of the mean (SEM). For panels A-C, age- and sex-matched data were analyzed using 1-way analysis of variance (ANOVA) with Tukey post hoc test. For panel D, sex-matched data were analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001 for the indicated comparisons.
At 8 weeks, hepcidin levels were similar in Bmp5se mice and sex-matched Bmp5wt controls (Figure 1A, right). In addition, there were no significant differences in serum iron, serum transferrin saturation, liver iron, or CBC parameters, except for mildly increased mean cell hemoglobin in Bmp5se females (supplemental Figure 1D, right; Figure 1B-C, right; supplemental Table 2). Together, these results suggest that BMP5 contributes to hepcidin expression in 10-day-old mice but is redundant in adult mice under basal conditions.
To understand the differences between 10-day- and 8-week-old mice, we quantitated the relative liver mRNA abundance of Bmp5 and Bmp6 in wild-type mice at these ages. Females had similar liver Bmp5 and Bmp6 levels at 10 days, whereas males had modestly higher Bmp6 (Figure 1D). In both sexes, liver Bmp6 was significantly higher at 8 weeks than at 10 days (Figure 1D), mirroring an increase in liver iron (Figure 1C). In contrast, Bmp5 did not increase with age (Figure 1D). Together, these data suggest that BMP5 plays a more important role in hepcidin regulation at earlier ages when liver Bmp6 mRNA levels are lower. In older mice, BMP6 plays a dominant role, likely because it is upregulated by iron loading.14,17
Bmp5-mutant mice exhibit mild liver iron loading under low- and high-iron conditions and impaired hepcidin responses to oral iron gavage
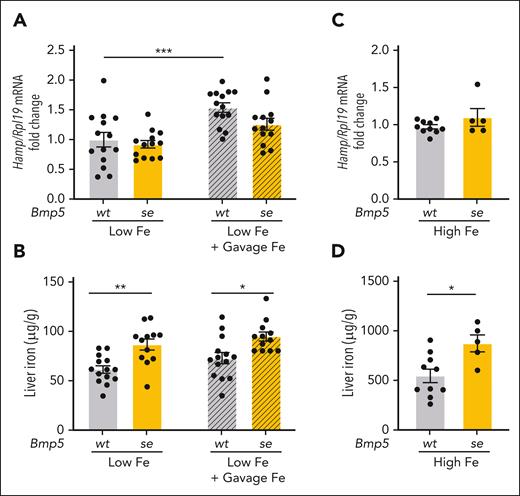
Next, we investigated whether BMP5 contributes to hepcidin and iron homeostasis regulation in adult mice when BMP6 levels are limited. A low-iron diet suppresses liver Bmp6 mRNA expression.14 Therefore, we examined the iron phenotype in Bmp5se-mutant mice and littermate Bmp5wt controls fed on a low-iron diet or a matched adequate-iron diet for 10 days. Analogous to the chow diet–fed mice (Figure 1; supplemental Figure 1), the iron phenotype was similar between adult Bmp5se-mutant mice and Bmp5wt mice fed on an adequate-iron diet (supplemental Figure 2). However, in the low-iron diet-fed mice, although liver Hamp mRNA levels were similar between genotypes (Figure 2A, left), liver iron loading was increased in Bmp5se mice compared with Bmp5wt mice (Figure 2B, left).
Bmp5-mutant mice exhibit mild liver iron loading under low- and high-iron conditions and impaired hepcidin responses to oral iron gavage. (A-B) Seven-week-old female Bmp5wt and Bmp5se mice were fed on a low-iron diet (2-6 ppm iron). After 10 days, the mice were orally gavaged with distilled water (Low Fe) or 2 mg/kg elemental iron (in the form of ferrous sulfate; Low Fe + Gavage Fe). After 5 hours, the livers were analyzed for Hamp relative to Rpl19 mRNA via qRT-PCR (A). The average of Bmp5wt mice on a low-iron diet was set to 1. (B) Liver iron levels were analyzed using colorimetric assay. (C-D) Four-week-old female Bmp5wt and Bmp5se mice were maintained on a high-iron diet (High Fe, 1% carbonyl iron) for 3 weeks. (C) Liver Hamp mRNA relative to Rpl19 was analyzed via qRT-PCR. The average of Bmp5wt mice on a high-iron diet was set to 1. (D) Liver iron levels were analyzed using a colorimetric assay. For all graphs, individual data points are shown, and bars represent mean ± SEM. For panels A-B, ∗P < .05; ∗∗P < .01 for the indicated comparisons using 2-way ANOVA with Tukey post hoc test. For panels C-D, ∗P < .05 for Bmp5se relative to Bmp5wt mice, using Student t test.
Bmp5-mutant mice exhibit mild liver iron loading under low- and high-iron conditions and impaired hepcidin responses to oral iron gavage. (A-B) Seven-week-old female Bmp5wt and Bmp5se mice were fed on a low-iron diet (2-6 ppm iron). After 10 days, the mice were orally gavaged with distilled water (Low Fe) or 2 mg/kg elemental iron (in the form of ferrous sulfate; Low Fe + Gavage Fe). After 5 hours, the livers were analyzed for Hamp relative to Rpl19 mRNA via qRT-PCR (A). The average of Bmp5wt mice on a low-iron diet was set to 1. (B) Liver iron levels were analyzed using colorimetric assay. (C-D) Four-week-old female Bmp5wt and Bmp5se mice were maintained on a high-iron diet (High Fe, 1% carbonyl iron) for 3 weeks. (C) Liver Hamp mRNA relative to Rpl19 was analyzed via qRT-PCR. The average of Bmp5wt mice on a high-iron diet was set to 1. (D) Liver iron levels were analyzed using a colorimetric assay. For all graphs, individual data points are shown, and bars represent mean ± SEM. For panels A-B, ∗P < .05; ∗∗P < .01 for the indicated comparisons using 2-way ANOVA with Tukey post hoc test. For panels C-D, ∗P < .05 for Bmp5se relative to Bmp5wt mice, using Student t test.
Next, we tested the impact of short-term iron administration by oral gavage in mice maintained on the low-iron diet. Iron gavage similarly increased serum iron (supplemental Figure 3A) without affecting liver iron (Figure 2B), Bmp6, or Bmp2 mRNA levels (supplemental Figure 3B-C) in both genotypes. However, although iron gavage significantly increased liver Hamp mRNA in Bmp5wt controls, Hamp was not significantly induced in Bmp5se mice (Figure 2A). Although this difference was relatively small, it was highly reproducible in the 3 independent mouse cohorts.
We also tested the impact of long-term dietary iron loading in Bmp5se mice when BMP6 levels were reported to be maximally induced.17 Analogous to the low-iron diet mice, Bmp5se mice fed on a high-iron diet for 3 weeks exhibited similar liver Hamp mRNA levels, but more liver iron loading, compared with Bmp5wt controls (Figure 2C-D). We did not observe any differences in serum iron, extrahepatic iron, liver Bmp6, or liver Bmp2 expression in high-iron diet Bmp5se mice compared with Bmp5wt controls (supplemental Figure 3D-G). Together, these data suggest that Bmp5 mutant mice have impaired hepcidin responses resulting in modest liver iron loading under both low- and high-iron conditions, circumstances when BMP6 was either limited or already maximally induced.
To explore where BMP5 is produced and whether iron regulates BMP5 expression, we measured Bmp5 mRNA levels in different liver cell populations, total liver, and other tissues after various iron challenges. There was no preferential expression of Bmp5 in any liver cell population tested (supplemental Figure 4A). Total liver Bmp5 mRNA was not induced by long-term dietary iron loading, consistent with previous reports,20 by genetic iron overload due to Bmp6 KO, or by short-term oral iron gavage (supplemental Figure 4B-C). Moreover, long-term dietary iron loading did not induce Bmp5 mRNA in any of the other tissues tested (supplemental Figure 4D-K).
Bmp5 mutation exacerbates hepcidin deficiency and iron overload when Bmp6 is genetically limited
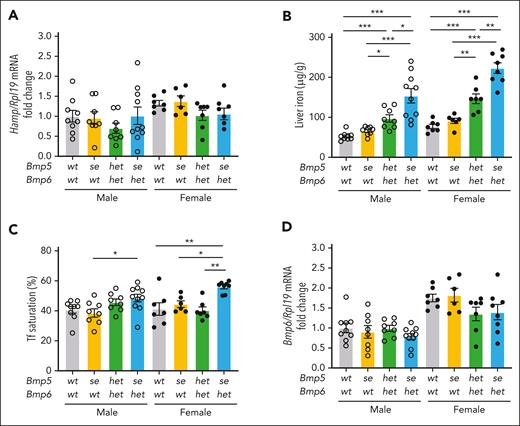
Next, we examined the role of BMP5 when BMP6 is genetically limited. Firstly, we investigated the impact of losing 1 or 2 functional Bmp5 alleles in Bmp6het mice (Bmp5het-Bmp6het or Bmp5se-Bmp6het) compared with littermate wild-type controls (Bmp5wt-Bmp6wt) and single Bmp5-mutant mice (Bmp5se-Bmp6wt). We did not collect single Bmp5wt-Bmp6het mice because we previously reported that Bmp6 heterozygosity had no impact on hepcidin or systemic iron homeostasis.16 Liver Hamp mRNA was not significantly changed in Bmp5het-Bmp6het or Bmp5se-Bmp6het mice compared with other genotypes (Figure 3A). However, liver iron progressively increased in the Bmp5het-Bmp6het and Bmp5se-Bmp6het mice (Figure 3B). Moreover, serum iron and transferrin saturation were increased in Bmp5se-Bmp6het females compared with other genotypes, and pancreas iron was increased in Bmp5se-Bmp6het vs wild-type males (Figure 3C; supplemental Figure 5A-B). There were no other significant differences in extrahepatic iron loading (supplemental Figure 5C-E). Despite losing 1 Bmp6 allele, liver Bmp6 mRNA was not reduced in Bmp5het-Bmp6het or Bmp5se-Bmp6het mice compared with Bmp6wt mice (Figure 3D). This is likely due to the increased liver iron loading in these mice (Figure 3B), which is known to upregulate Bmp6 mRNA.14,17 Liver Bmp2 mRNA expression was also similar among genotypes (supplemental Figure 5F). This inducibility of Bmp6 by iron may explain why Hamp levels in these mice seemed similar to those in wild-type controls, albeit at a higher setpoint of body iron loading.
Bmp5 deficiency causes mild iron loading in heterozygous Bmp6het mice. Eight-week-old male (open circles) and female (closed circles) Bmp5wt-Bmp6wt, Bmp5se-Bmp6wt, Bmp5het-Bmp6het, and Bmp5se-Bmp6het mice were analyzed for liver Hamp relative to Rpl19 mRNA using qRT-PCR (A). Liver iron (B) and Tf saturation levels (C) were analyzed using colorimetric assays. (D) Liver Bmp6 relative to Rpl19 mRNA was analyzed via qRT-PCR. For panels A and D, the average of male Bmp5wt-Bmp6wt mice was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 1-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗ P < .01; ∗∗∗ P < .001 for the indicated comparisons.
Bmp5 deficiency causes mild iron loading in heterozygous Bmp6het mice. Eight-week-old male (open circles) and female (closed circles) Bmp5wt-Bmp6wt, Bmp5se-Bmp6wt, Bmp5het-Bmp6het, and Bmp5se-Bmp6het mice were analyzed for liver Hamp relative to Rpl19 mRNA using qRT-PCR (A). Liver iron (B) and Tf saturation levels (C) were analyzed using colorimetric assays. (D) Liver Bmp6 relative to Rpl19 mRNA was analyzed via qRT-PCR. For panels A and D, the average of male Bmp5wt-Bmp6wt mice was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 1-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗ P < .01; ∗∗∗ P < .001 for the indicated comparisons.
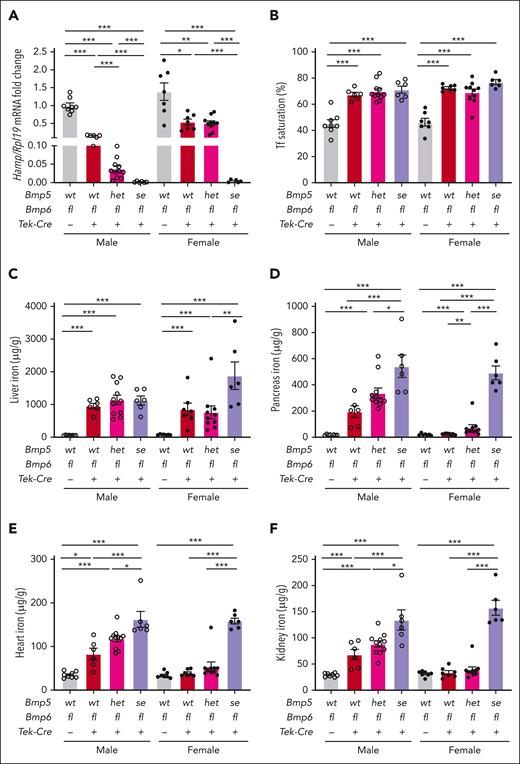
Next, we investigated the impact of losing 1 or 2 functional Bmp5 alleles in global Bmp6-KO mice (Bmp5se-Bmp6ko or Bmp5het-Bmp6ko) compared with littermate single Bmp6ko mice (Bmp5wt-Bmp6ko) and wild-type controls (Bmp5wt-Bmp6wt). Because we noticed increased mortality in double global mutant mice, we also generated endothelial Bmp6 KO mice (Bmp6fl-Tek-Cre+) with 0 to 2 functional Bmp5 alleles. As demonstrated previously,9,10,16 liver Hamp expression was significantly reduced, and serum iron, serum transferrin saturation, and liver iron were increased in Bmp6fl-Tek-Cre+ and Bmp6ko mice compared with the respective Cre– or wild-type controls (Figure 4A-C; supplemental Figures 6A, 7A-D, and 8). Bmp6fl-Tek-Cre+ and Bmp6ko males also exhibited extrahepatic iron loading in the pancreas, heart, and kidney, whereas extrahepatic iron loading was largely absent in Bmp6fl-Tek-Cre+ and Bmp6ko females (Figure 4D-F; supplemental Figures 7F-H and 8). Bmp6fl-Tek-Cre+ mice and Bmp6ko females exhibited reduced spleen iron, although no significant changes were observed in Bmp6ko males (supplemental Figures 6C, 7I, and 8). No compensatory increases in liver Bmp2 mRNA were observed (supplemental Figures 6D and 7J), as previously reported.20
Bmp5 deficiency exacerbates hepcidin deficiency and iron overload in endothelial Bmp6-KO mice. Eight-week-old male (open circles) and female (closed circles) Bmp5wt-Bmp6fl-Tek-Cre–, Bmp5wt-Bmp6fl-Tek-Cre+, Bmp5het-Bmp6fl-Tek-Cre+-, and Bmp5se-Bmp6fl-Tek-Cre+ mice were analyzed for liver Hamp relative to Rpl19 mRNA via qRT-PCR (A). The average of male Bmp5wt-Bmp6fl-Tek-Cre– mice was set to 1. Tf saturation (B), liver iron (C), pancreas iron (D), heart iron (E), and kidney iron levels (F) were analyzed using colorimetric assays. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 1-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for the indicated comparisons.
Bmp5 deficiency exacerbates hepcidin deficiency and iron overload in endothelial Bmp6-KO mice. Eight-week-old male (open circles) and female (closed circles) Bmp5wt-Bmp6fl-Tek-Cre–, Bmp5wt-Bmp6fl-Tek-Cre+, Bmp5het-Bmp6fl-Tek-Cre+-, and Bmp5se-Bmp6fl-Tek-Cre+ mice were analyzed for liver Hamp relative to Rpl19 mRNA via qRT-PCR (A). The average of male Bmp5wt-Bmp6fl-Tek-Cre– mice was set to 1. Tf saturation (B), liver iron (C), pancreas iron (D), heart iron (E), and kidney iron levels (F) were analyzed using colorimetric assays. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 1-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for the indicated comparisons.
Losing 1 functional Bmp5 allele in Bmp5het-Bmp6fl-Tek-Cre+ or Bmp5het-Bmp6ko mice further suppressed liver Hamp expression in males and increased extrahepatic iron overload in Bmp5het-Bmp6ko and, to a lesser extent, in Bmp5het-Bmp6fl-Tek-Cre+ females (Figure 4A,D-F; supplemental Figures 7A,E-H and 8). Loss of both functional Bmp5 alleles in Bmp5se-Bmp6fl-Tek-Cre+ and Bmp5se-Bmp6ko mice dramatically downregulated Hamp expression compared with other genotypes (Figure 4A; supplemental Figure 7A), although the increased mortality in Bmp5se-Bmp6ko mice precluded a statistical comparison in females, among which only 2 survived for analysis. Although the cause of mortality is unclear, there were no gross abnormalities in liver histology (supplemental Figure 9). Hamp deficiency resulted in increased tissue iron loading in double mutant Bmp5se-Bmp6fl-Tek-Cre+ and Bmp5se-Bmp6ko mice compared with that in other genotypes, although we did not, for the most part, observe further increases in serum iron or transferrin saturation, which were already very elevated (Figure 4B-F; supplemental Figures 6A, 7B-H, and 8). There was no compensatory induction of liver Bmp2 (supplemental Figures 6D and 7J).
Hepcidin expression is not induced by iron in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice
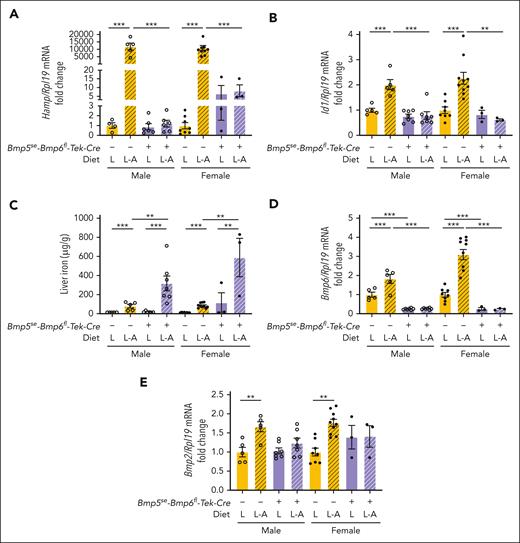
We previously reported that long-term dietary iron loading robustly induced hepcidin expression (∼500-fold) in global and endothelial Bmp6-KO mice, and this was not affected by endothelial Bmp2 KO.12,15,20 Therefore, we investigated whether BMP5 contributes to iron-mediated hepcidin induction in endothelial Bmp6-KO mice. To avoid iron overload caused by a standard rodent diet (Figure 4), double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice were weaned to a low-iron diet (2-6 ppm iron) for 3 weeks and either kept on the low-iron diet or switched to a matched, purified adequate-iron diet (48 ppm iron) for 1 week. The results were compared with those of littermate single Bmp5se-Bmp6fl-Tek-Cre– mutant mice, which have largely preserved hepcidin responses to long-term dietary iron loading (Figures 1-2). As expected, liver Hamp was dramatically induced by iron in single Bmp5se-Bmp6fl-Tek-Cre– mutant mice, together with the SMAD1/5/8 target transcript Id1 (Figure 5A-B, yellow bars). However, Hamp and Id1 were not induced by iron in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice (Figure 5A-B, purple bars). Although serum transferrin saturation was similarly induced by the adequate-iron diet in both genotypes (supplemental Figure 10A), the failure to induce hepcidin resulted in greater liver iron loading in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice of both sexes (Figure 5C) and extrahepatic iron loading in females (supplemental Figure 10B-D). Liver Bmp6 and Bmp2 expressions were significantly induced by iron in single Bmp5se-Bmp6fl-Tek-Cre– mutant mice (Figure 5D-E) but not in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice, consistent with endothelial Bmp6 gene deletion and a prior report that liver Bmp2 is not induced by iron in Bmp6-KO mice.20 Together, these data demonstrate that BMP5 is required for the entirety of iron signaling to hepcidin in the absence of BMP6.
Hepcidin expression is not inducible by iron in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice. Three-week-old male (open circles) and female (closed circles) double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice and littermate single Bmp5-mutant mice (Bmp5se-Bmp6fl-Tek-Cre-) were maintained on a low-iron diet (2-6 ppm iron) at weaning for 3 weeks to prevent iron overload induced by a standard rodent diet. Mice were either maintained on a low-iron diet (L) or switched to a matched purified adequate-iron diet (L-A; 48 ppm iron) for 1 more week. Mice were analyzed for liver Hamp (A) and Id1 relative to Rpl19 mRNA (B) via qRT-PCR. (C) Liver iron was analyzed using a colorimetric assay. Liver Bmp6 (D) and Bmp2 relative to Rpl19 mRNA (E) were analyzed via qRT-PCR. For panels A-B and D-E, the average of single Bmp5 mutant male mice on the low-iron diet was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for the indicated comparisons.
Hepcidin expression is not inducible by iron in double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice. Three-week-old male (open circles) and female (closed circles) double Bmp5se-Bmp6fl-Tek-Cre+ mutant mice and littermate single Bmp5-mutant mice (Bmp5se-Bmp6fl-Tek-Cre-) were maintained on a low-iron diet (2-6 ppm iron) at weaning for 3 weeks to prevent iron overload induced by a standard rodent diet. Mice were either maintained on a low-iron diet (L) or switched to a matched purified adequate-iron diet (L-A; 48 ppm iron) for 1 more week. Mice were analyzed for liver Hamp (A) and Id1 relative to Rpl19 mRNA (B) via qRT-PCR. (C) Liver iron was analyzed using a colorimetric assay. Liver Bmp6 (D) and Bmp2 relative to Rpl19 mRNA (E) were analyzed via qRT-PCR. For panels A-B and D-E, the average of single Bmp5 mutant male mice on the low-iron diet was set to 1. For all graphs, individual data points are shown, and bars represent mean ± SEM. Sex-matched data were analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for the indicated comparisons.
ERFE binds to BMP5 and inhibits BMP5 signaling to hepcidin
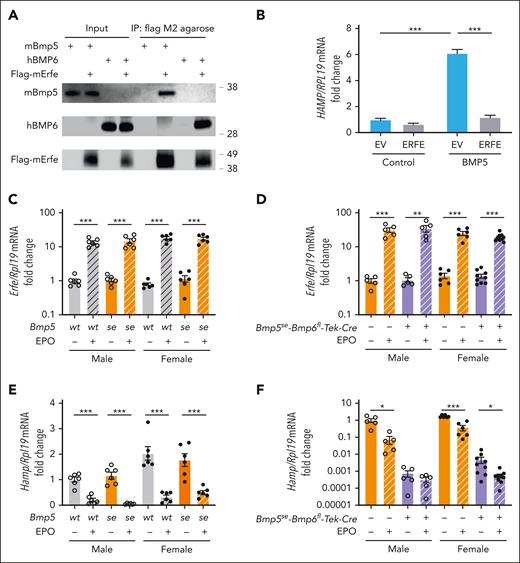
ERFE acts as a ligand trap that sequesters BMPs from binding to the BMP receptor complex to induce hepcidin.23 As previously reported,24 we found that induction of endogenous Erfe by EPO injection suppressed liver Hamp mRNA in single Bmp6ko mice (supplemental Figure 11). Therefore, we tested whether targeting BMP5 is 1 mechanism by which ERFE suppresses hepcidin expression. ERFE bound directly to BMP5, similar to BMP6, via coimmunoprecipitation (Figure 6A). Moreover, ERFE inhibited BMP5 induction of HAMP mRNA in Hep3B cells (Figure 6B). In vivo, EPO treatment similarly induced Erfe in all mouse genotypes (Figure 6C-D). However, although EPO treatment similarly suppressed liver Hamp in single Bmp5se-mutant mice compared with wild-type mice (Figure 6E), EPO failed to suppress Hamp in double Bmp5se-Bmp6fl-Tek-Cre+ mutant males, although there was some residual Hamp suppression in double mutant females in which basal Hamp levels were higher (Figure 6F).
ERFE binds to BMP5 and inhibits BMP5 signaling to hepcidin. (A) BMP5 (2 μg) or BMP6 (1 μg) was mixed with 1 μL of Flag-ERFE concentrate (52.9 ng/μL) supplemented with a protease inhibitor cocktail. After saving 3% of the total mixture as input, the solutions were incubated with 10 μL of Flag M2 agarose at 4°C overnight. The immunoprecipitated complexes were washed and eluted with 150 μg/mL of 3× Flag peptide in tris-buffered saline for 30 minutes at 4°C. The inputs and eluents were immunoblotted using antibodies against BMP5, BMP6, and Flag. The experiments were repeated 3 times, with 1 representative blot shown. (B) Hep3B cells were transfected with Flag-ERFE or an empty vector (EV). After 48 hours, cells were treated with 5 ng/μL BMP5 for 6 hours. HAMP relative to RPL19 mRNA was analyzed via qRT-PCR. (C-F) Eight-week-old male (open circles) and female (closed circles) single Bmp5se and littermate control Bmp5wt mice (C,E) or double Bmp5se-Bmp6fl-Tek-Cre+ and littermate control Bmp5se-Bmp6fl-Tek-Cre– mice (D,F) were given 200 units of Epogen (EPO) or phosphate buffered saline (PBS) intraperitoneally. After 15 hours, bone marrow Erfe (C,D) and liver Hamp (E,F) relative to Rpl19 mRNA were analyzed via qRT-PCR. The average of male Bmp5wt or Bmp5se-Bmp6fl-Tek-Cre– control mice treated with PBS was set to 1. For panel B, bars represent the mean ± SEM from 4 independent experiments. ∗∗∗P < .001 for the indicated comparisons by 2-way ANOVA with Tukey post hoc test. For panels C-F, individual data points are shown, and bars represent the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for EPO relative to PBS treatment for sex- and genotype-matched mice using Student t test.
ERFE binds to BMP5 and inhibits BMP5 signaling to hepcidin. (A) BMP5 (2 μg) or BMP6 (1 μg) was mixed with 1 μL of Flag-ERFE concentrate (52.9 ng/μL) supplemented with a protease inhibitor cocktail. After saving 3% of the total mixture as input, the solutions were incubated with 10 μL of Flag M2 agarose at 4°C overnight. The immunoprecipitated complexes were washed and eluted with 150 μg/mL of 3× Flag peptide in tris-buffered saline for 30 minutes at 4°C. The inputs and eluents were immunoblotted using antibodies against BMP5, BMP6, and Flag. The experiments were repeated 3 times, with 1 representative blot shown. (B) Hep3B cells were transfected with Flag-ERFE or an empty vector (EV). After 48 hours, cells were treated with 5 ng/μL BMP5 for 6 hours. HAMP relative to RPL19 mRNA was analyzed via qRT-PCR. (C-F) Eight-week-old male (open circles) and female (closed circles) single Bmp5se and littermate control Bmp5wt mice (C,E) or double Bmp5se-Bmp6fl-Tek-Cre+ and littermate control Bmp5se-Bmp6fl-Tek-Cre– mice (D,F) were given 200 units of Epogen (EPO) or phosphate buffered saline (PBS) intraperitoneally. After 15 hours, bone marrow Erfe (C,D) and liver Hamp (E,F) relative to Rpl19 mRNA were analyzed via qRT-PCR. The average of male Bmp5wt or Bmp5se-Bmp6fl-Tek-Cre– control mice treated with PBS was set to 1. For panel B, bars represent the mean ± SEM from 4 independent experiments. ∗∗∗P < .001 for the indicated comparisons by 2-way ANOVA with Tukey post hoc test. For panels C-F, individual data points are shown, and bars represent the mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for EPO relative to PBS treatment for sex- and genotype-matched mice using Student t test.
Discussion
The BMP-SMAD pathway is central to hepcidin transcriptional regulation in response to iron and erythropoietic drive.8 BMP6 and BMP2 are key endogenous hepcidin regulators; however, iron and erythropoiesis still regulate hepcidin in mice lacking 1 or both ligands.9-12,15,20 Here, we examined the iron phenotype of mice with inactivating Bmp5 mutations, either alone or together with Bmp6 KO, to demonstrate the functional role of BMP5 in hepcidin and iron homeostasis regulation.
We showed that under basal conditions, Bmp5se mice exhibited hepcidin deficiency at an early age but apparently normal hepcidin levels and systemic iron homeostasis parameters in adulthood. However, Bmp5se mice were prone to liver iron loading when challenged with low- or high-iron diets and exhibited blunted hepcidin responses to enteral iron under low-iron conditions. This contrasts with Bmp6- and/or endothelial Bmp2-KO mice, which exhibit profound hepcidin deficiency throughout life and progressive serum and tissue iron overload when fed on a standard diet.9-12,16 These data suggest that BMP5 has a functional role in hepcidin and iron homeostasis regulation, but its role is relatively minor compared with that of BMP6 and BMP2.
We attribute the relatively minor role of BMP5 to the fact that liver Bmp6 and, to a lesser extent, Bmp2 mRNA are increased by iron,14,17,20 whereas Bmp5 mRNA was not regulated by iron in any tissues or experimental conditions tested. According to this model, hepcidin deficiency induced by Bmp5 mutations would increase dietary iron absorption, which would upregulate liver Bmp6 and Bmp2, thereby increasing hepcidin and preventing iron overload. This may explain why Bmp5se mice had hepcidin deficiency at 10 days but no apparent iron phenotype at 8 weeks on a standard diet. In support of this model, quantitative copy number analysis helped confirm increased liver Bmp6 mRNA in 8-week-old compared with that in 10-day-old mice. However, under conditions when Bmp6 and Bmp2 are limited, for example, in mice fed on a low-iron diet, there was a larger functional impact of Bmp5 mutation, resulting in blunted hepcidin responses to iron and mild liver iron loading. Our data also demonstrated a greater impact of BMP5 deficiency in mice fed on a high-iron diet for 3 weeks. Based on this data and our previous finding that Bmp6 mRNA induction reaches a peak after 2 weeks of dietary iron loading,17 we hypothesize that Bmp6 is unable to fully compensate for the loss of Bmp5 in this setting because it is already maximally induced.
Although our data are consistent with a model in which BMP6 and BMP2 largely compensate for the loss of BMP5 in hepcidin regulation due to their inducibility by iron, this does not preclude other explanations. For example, the cellular source of BMP5, BMP5 protein levels, and/or the nature of BMP5 interactions with the BMP receptor complex or accessory molecules may contribute to BMP5’s less potent role. Future studies are required to explore these potential contributory factors.
Our data demonstrated a more important role for BMP5 when BMP6 is genetically limited. Indeed, Bmp5se-Bmp6het mice had increased serum iron (in females) and liver iron (in both sexes) compared with Bmp5het-Bmp6het mice, which exhibited increased liver iron compared with Bmp5wt-Bmp6wt controls. Because we did not collect Bmp5wt-Bmp6het mice in this study, we could not definitively determine whether Bmp5het-Bmp6het mice had higher iron loading than Bmp5wt-Bmp6het mice; however, we previously reported that Bmp6het mice did not exhibit iron loading compared with Bmp6wt mice.16 Although absolute hepcidin levels were unchanged in Bmp5se-Bmp6het and Bmp5het-Bmp6het mice, hepcidin levels were inappropriately low relative to the degree of iron loading, which should normally induce hepcidin expression.3,14,17 Indeed, although not a perfect measure because it does not account for changes in extrahepatic or serum iron, the ratio of hepcidin to liver iron was significantly reduced in both Bmp5het-Bmp6het and Bmp5se-Bmp6het mice relative to Bmp5wt-Bmp6wt controls (supplemental Figure 5G). Notably, most genetic mutations that cause hemochromatosis, including HFE, HJV, and TFR2, are associated with defective BMP-SMAD signaling.33-39 Moreover, hemochromatosis has variable penetrance and the genetic factors that account for this are not fully understood.40 Our data raise the possibility that Bmp5 mutations could function as a modifier of disease penetrance in patients with mutations in other known hemochromatosis genes.
The impact of Bmp5 mutations was even more pronounced in global or endothelial Bmp6-KO mice, in which there was a dramatic hepcidin reduction and increased extrahepatic iron overload. Moreover, although we previously reported that iron still robustly induced hepcidin (∼500-fold) in mice lacking either Bmp6 alone or Bmp6 together with endothelial Bmp2,20 double Bmp5- and Bmp6-mutant mice completely failed to induce hepcidin in response to dietary iron loading. These data demonstrated that BMP6-independent hepcidin induction by iron is fully dependent on BMP5. How iron enhances hepcidin expression via BMP5 is still uncertain. Iron did not increase Bmp5 mRNA in the liver or other tissues, rather Bmp5 was reduced in some tissues for uncertain reasons. It is possible that iron increases BMP5 protein expression, secretion, and/or availability through posttranscriptional mechanisms. It is also possible that iron leads to enhanced sensitivity of the downstream SMAD signaling pathway response to BMP5 ligand, for example, by making the BMP receptor complex more responsive, as previously reported for the coreceptor hemojuvelin.35
Although our data support a functional role of BMP5 in hepcidin and iron homeostasis regulation, they do not rule out the contributory role of other BMP ligands. Indeed, BMP6 was the first discovered hepcidin regulator9,10 but was subsequently shown to work together with BMP2, as demonstrated by an identical hemochromatosis phenotype in mice lacking either endothelial Bmp2 or Bmp6 or both.20 BMPs are known to function as homodimers or heterodimers, and it is hypothesized that BMP2 and BMP6 regulate hepcidin as a heterodimeric ligand.20 It is possible that BMP5 may similarly work together with another BMP ligand. Future studies are needed to understand whether any other BMP ligands contribute to hepcidin regulation together with BMP5.
EPO injection suppressed hepcidin in single Bmp6ko or Bmp5se mice but not in double Bmp5se-Bmp6fl/Tek-Cre+ mutant males, in which basal hepcidin expression was much lower. Mechanistically, ERFE bound directly to BMP5 and inhibited hepcidin induction by BMP5, similar to ERFE effects on BMP6 and BMP2/6.23 Our data suggest that EPO lowers hepcidin in Bmp6ko mice by inhibiting BMP5. Additionally, EPO lowers hepcidin in Bmp5se mice by inhibiting BMP6 and/or BMP2/6. We hypothesize that the lack of EPO effect in double Bmp5seBmp6fl/Tek-Cre+ mutant males is due to insufficient BMP ligand to signal hepcidin, which may already be at nadir. Interestingly, EPO still suppressed hepcidin in double Bmp5seBmp6fl/Tek-Cre+ mutant females, in which basal hepcidin expression was higher. The reasons for this are unclear but may be due to mild residual BMP6 and/or BMP2/6 signaling in endothelial Bmp6-KO mice, either from <100% Cre recombination efficiency or a minor contribution from another cellular source.16 This may be observed only in females because the low level of residual BMP6 and/or BMP2/6 ligand may not be sufficient to induce hepcidin in males, in which testosterone has been reported to interfere with SMAD1/5 signaling.41,42 There may also be a contributory role of another BMP ligand.
In conclusion, our results reveal a novel functional role for BMP5 in hepcidin regulation and system iron homeostasis, particularly under conditions in which BMP6 is limiting. These results provide new insights into how the liver integrates iron and erythropoietic signals to control hepcidin regulation and systemic iron homeostasis.
Acknowledgments
This work was supported by a MGH ECOR Fund for Medical Discovery Fundamental Research Fellowship Award (X.X.), National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases grants R01-DK128068 (J.L.B. and J.C.), R01-DK087727 (J.L.B.), and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award (J.L.B.).
Authorship
Contribution: X.X. and Y.X. designed and performed the experiments, interpreted the data, and wrote the manuscript; G.A.M., Y.Y., A.L.F., V.M.A.-M., S.M., S.P., and C.-Y.W. assisted with mouse studies and edited the manuscript; J.C. acquired funding, provided critical discussions, and edited the manuscript; and J.L.B. conceived and oversaw the study, acquired funding, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.L.B. has been a consultant for the Incyte Corporation and Alnylam Pharmaceuticals; owns equity in Ferrumax Pharmaceuticals, a company focused on targeting RGM proteins (including hemojuvelin) and bone morphogenetic protein (BMP/TGF-beta) superfamily signaling as hepcidin-modulating agents for the treatment of anemia and other iron disorders; and has interests reviewed and managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
Correspondence: Jodie L. Babitt, Massachusetts General Hospital, Harvard Medical School, Thier Research Bldg, 1123A, 50 Blossom St, Boston, MA 02114; e-mail: babitt.jodie@mgh.harvard.edu.
References
Author notes
∗X.X and Y.X. contributed equally to this work.
Data are available on request from the corresponding author, Jodie L. Babitt (babitt.jodie@mgh.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal